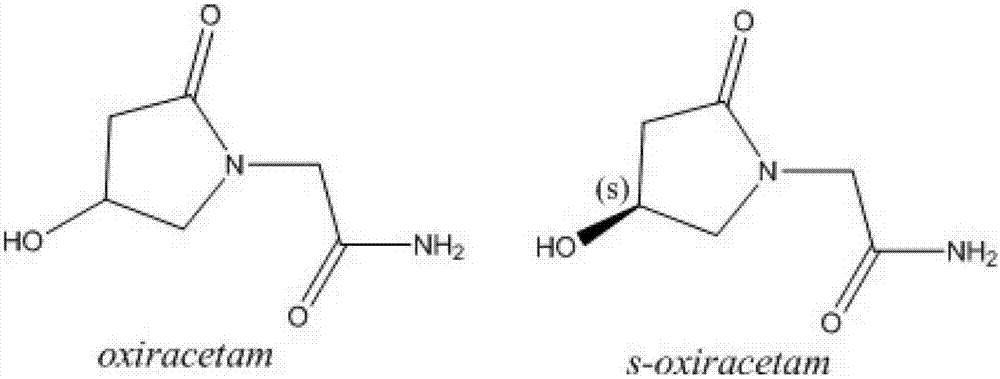
(S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide effervescent tablets having good stability and preparation method of effervescent tablets
A technology of pyrrolidine acetamide and stability, which is applied in the field of -4-hydroxy-2-oxo-1-pyrrolidine acetamide effervescent tablet and its preparation, and can solve the problem that the particle size is not easy to control, the particle is not easy to form, and the particle powder is not easy to control. Problems such as multiple layers, etc., to achieve the effect of simple and feasible preparation process, not easy to absorb moisture and deteriorate, and good particle formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A stable (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide effervescent tablet is prepared according to the following steps:
[0025] Element Dosage (S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide 1 copy tartaric acid 5 copies sodium bicarbonate 5 copies Sodium chloride 0.5 parts polyethylene glycol 6000 0.75 parts dextrin 5 copies sucrose 5 copies 8% mass fraction of PVP / VA64 ethanol solution 17 copies Sodium carboxymethyl cellulose 5 copies microcrystalline cellulose 8 servings
[0026] Made into 1000 pieces
[0027] Preparation process:
[0028]1. Take the prescribed amount of (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, tartaric acid, dextrin, sucrose, sodium carboxymethyl cellulose, microcrystalline cellulose and mix them in a universal pulverizer During, pulverize, collect the mixed powder after passing through a 100-mesh sieve, put the mixed powder in a wet granulator, add a binder, start t...
Embodiment 2
[0072] A stable (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide effervescent tablet is prepared according to the following steps:
[0073] Element Dosage (S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide 1 copy tartaric acid 7 copies sodium bicarbonate 7 copies Sodium chloride 0.8 parts polyethylene glycol 6000 1.2 parts dextrin 8 servings sucrose 7 copies 12% mass fraction of PVP / VA64 ethanol solution 23 copies Sodium carboxymethyl cellulose 8 servings microcrystalline cellulose 11 copies
[0074] Made into 1000 pieces
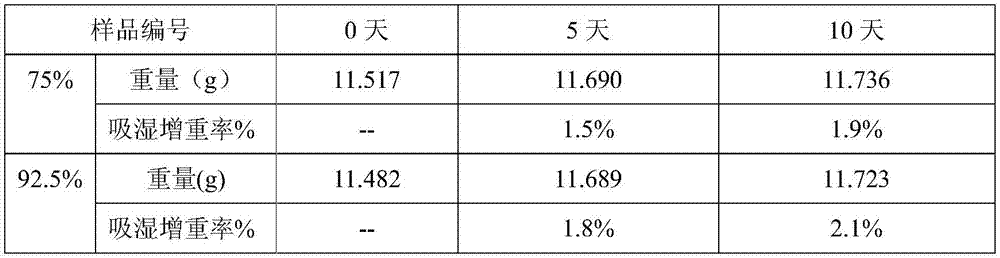
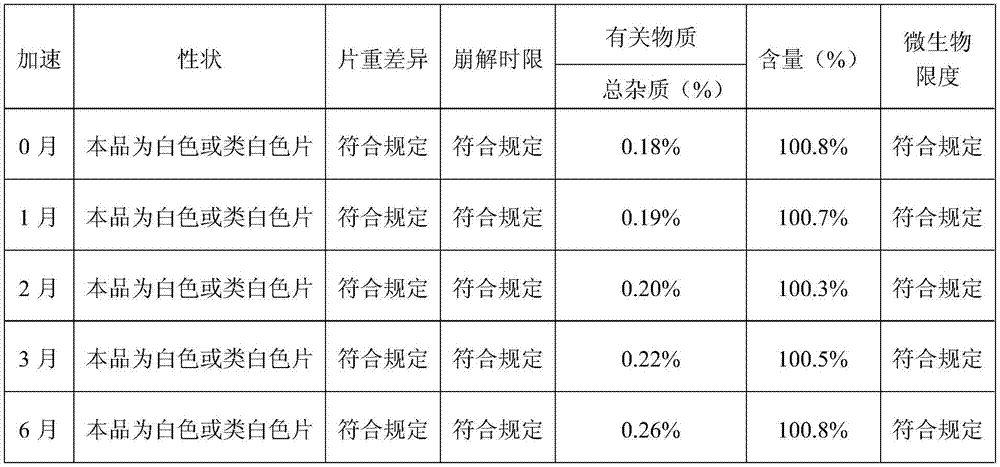
[0075] Preparation process: prepared according to the preparation process of Example 1. According to the test method of Example 1, the measurement of the particle size of the preparation process, the measurement of the moisture absorption and weight gain of the finished product, the inspection of the disintegration time limit and the stability test of the sample were carried out respect...
Embodiment 3
[0077] A stable (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide effervescent tablet is prepared according to the following steps:
[0078] Element Dosage (S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide 1 copy tartaric acid 6 servings sodium bicarbonate 6 servings Sodium chloride 0.7 parts polyethylene glycol 6000 1.05 parts dextrin 6 servings sucrose 6 servings 12% mass fraction of PVP / VA64 ethanol solution 21 copies Sodium carboxymethyl cellulose 7 copies microcrystalline cellulose 10 copies
[0079] Made into 1000 pieces
[0080] Preparation process: prepared according to the preparation process of Example 1. According to the test method of Example 1, the measurement of the particle size of the preparation process, the measurement of the moisture absorption and weight gain of the finished product, the inspection of the disintegration time limit and the stability test of the sample were carried out re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com