Anti-IL-1beta monoclonal antibody and application thereof
A monoclonal antibody and sequence technology, applied in applications, antibodies, anti-inflammatory agents, etc., can solve the problems of reduced gout attack rate and high incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
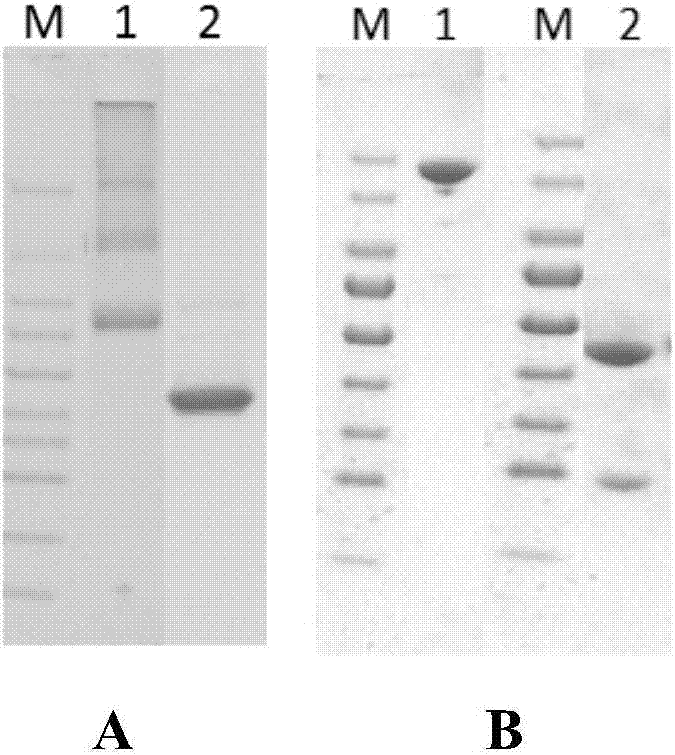
[0056] The specific anti-IL-1β monoclonal antibody preparation and related detection methods are as follows: Construction of IL-1β fully human antibody library, including optimization of phage display vectors, isolation of PBMC cells, RNA extraction, cDNA reverse transcription and antibody genes Amplification and enzyme digestion, ligation, electroporation, etc.; screening of antibodies, including antigen preparation, phage display screening, ELISA screening, etc.; construction of full-length antibodies, expression, purification, and affinity determination, cell activity determination, etc.
[0057] The anti-IL-1β monoclonal antibody provided by the present invention has a heavy chain variable region and a light chain variable region:
[0058] (I) The amino acid sequence of the heavy chain variable region is shown in any one of SEQ ID No: 1-6; or one or more amino acids are substituted, deleted or added; or the amino acid sequence shown in (I) Amino acid sequences with at leas...
Embodiment 1
[0154] The preparation of embodiment 1 antigenic protein
[0155] 1. Antigen and positive antibody gene synthesis and expression vector construction:
[0156] The amino acid sequence design of fusion human IL-1β mature protein amino acid sequence and connecting peptide-mIgG1Fc is shown in SEQ ID NO:25. The amino acid sequence design of fusion human IL-1β mature protein amino acid sequence and connecting peptide-6his is shown in SEQ ID NO:26.
[0157] The amino acid sequence corresponding to the human IL-1β mature protein fusion protein designed above (IL-1β-linked peptide-mIgG1Fc or IL-1β-linked peptide-6his) is artificially optimized for codons, and the codon-optimized sequence is shown as SEQ ID NO: 27 or 28 add Hind III restriction site and Kozak sequence GCCGCCACC at the 5' end, add stop codon TAG and EcoR I restriction site at the 3' end, and entrust Suzhou Synbio Company to synthesize the optimized DNA , cloned into pUC57simple (provided by Suzhou Synbio) vector to obt...
Embodiment 2
[0169] Example 2: Construction of natural human single chain antibody phage display library
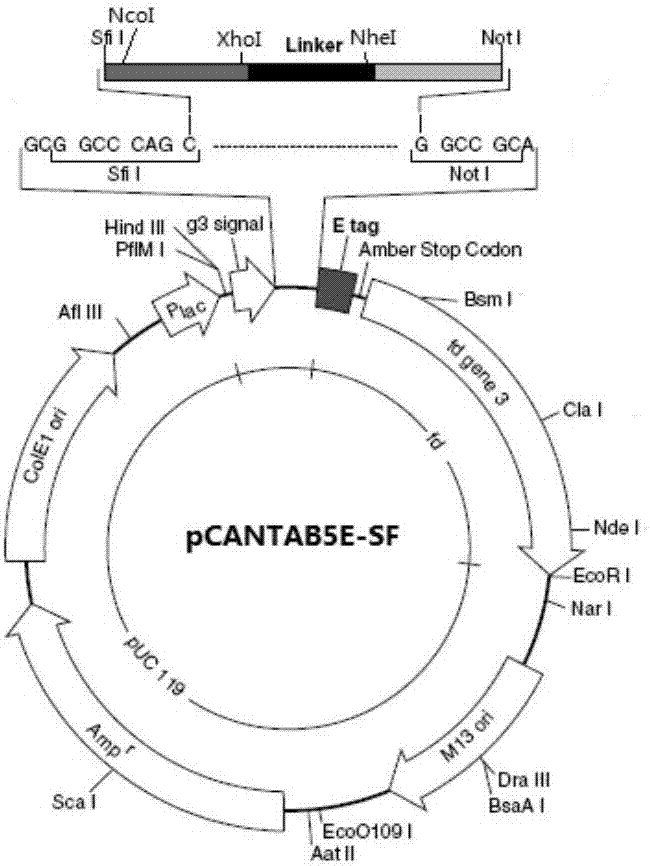
[0170] 1. Construction of phagemid vector
[0171] Select pCANTAB5E as the phage display vector, and carry out vector transformation according to the needs of cloning and phage display. The transformation results are as follows: figure 2 . The SfiI-NcoI-XhoI+Linker+NheI-NotI sequence (SEQ ID NO: 31) was gene-synthesized, then digested with SfiI and NotI, and ligated with the pCANTAB5E vector for recombinant construction to obtain the transformed vector pCANTAB5E-SF.
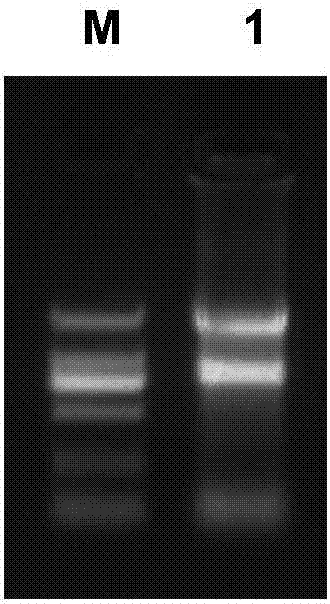
[0172] 2. PBMC isolation and mRNA extraction
[0173] Aseptically extract fresh peripheral blood from healthy volunteers, use lymphocyte separation medium (GE) to separate the lymphocytes therein, and use Invitrogen’s reagent (15596-026) extracts 100×10 6 The total RNA of each cell, the result is as follows image 3 shown.
[0174] 3. Antibody library primer design, synthesis and RT-PCR
[0175] According to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com