PLA-PEG-PLA dendritic polymer
A PLA-PEG-PLA, dendritic technology, applied in the field of medicinal chemistry, can solve the problems of limited choice, uncertainty, and difficulty in obtaining approval, and achieve the effect of tight micellar structure, improved stability and good targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
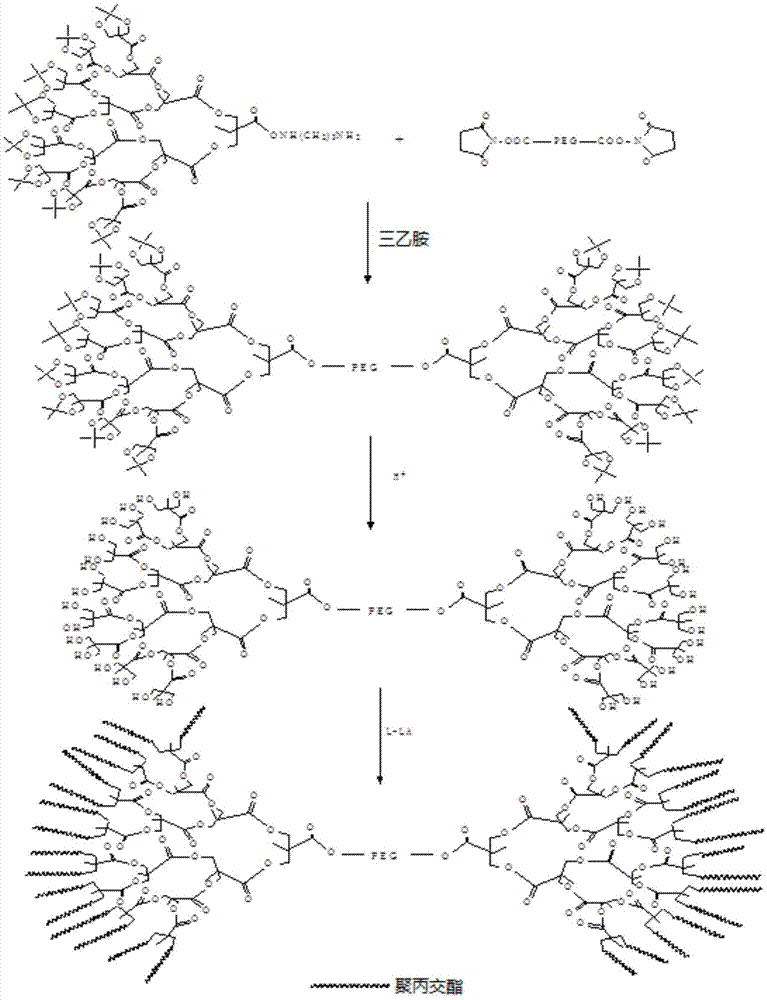
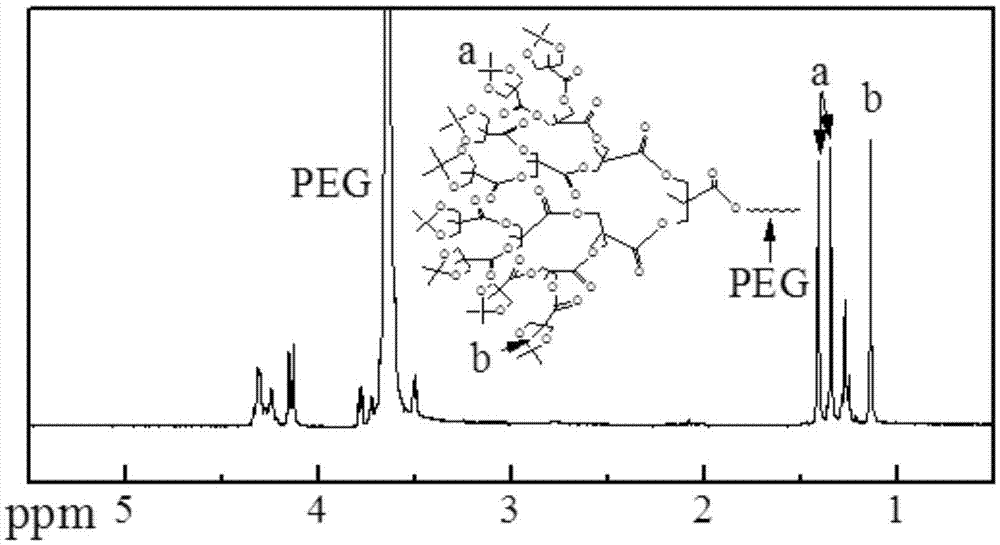
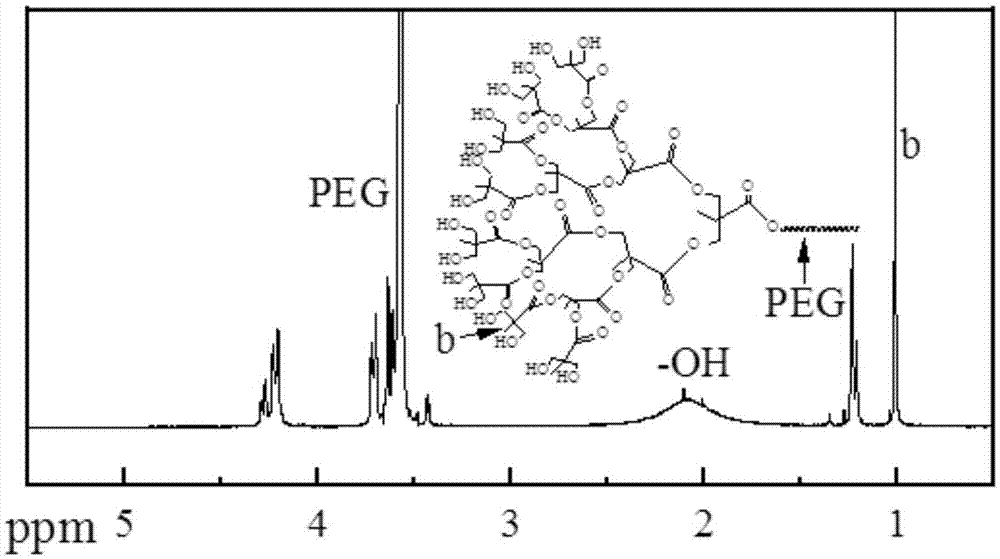
[0039] Embodiment 1 synthesizes the PLA-PEG-PLA dendritic polymer of 32 hydroxyls
[0040] Its specific reaction is as figure 1 shown.
[0041] (1) Synthesis of NHS-capped polyethylene glycol (NHS-PEG-NHS)
[0042] Dissolve 10 g of carboxypolyethylene glycol (molecular weight: 2000) in 50 mL of anhydrous dichloromethane, add 1.16 g of NHS, 10 mmol), 2.08 g of cyclohexylcarbodiimide (DCC), and 0.12 g of dichloromethane Methylolpropionic acid (bis-MPA) and 1.1 g of 4-dimethylaminopyridine-p-toluenesulfonate (DPTS) were stirred and reacted at room temperature for 48 hours to obtain a reaction solution. After filtering the reaction solution to remove insoluble matter, pour it into 1L ether for precipitation, redissolve the precipitate in an organic solvent, such as tetrahydrofuran, ethyl acetate, dichloromethane or chloroform, etc., and then remove the water-insoluble impurities produced by side reactions , preferably by centrifugation to remove the precipitate and re-precipita...
Embodiment 2
[0056] Example 2 Benzyl-terminated copolymer encapsulated paclitaxel
[0057] Add 5 g of the dendritic LA-PEG-PLA dendritic polymer obtained in the first step and 2 g of benzoyl chloride into a round-bottomed flask, heat it to 100 ° C for 6 h under nitrogen protection, and wash the product with dichloromethane after cooling to room temperature Dissolved and ether precipitated to obtain a white powdery solid, which is the benzyl-terminated copolymer excipient Bz endcapped mPEG-PLA.
[0058] Dissolve 1 g of the benzyl-terminated polymer excipient obtained in the above steps and an appropriate amount of paclitaxel in ethyl acetate, remove the solvent by rotary evaporation at 50 ° C, add 50 ml of water for injection to dissolve, and filter the solution through a 0.22 μm PVDF membrane to remove uncoated paclitaxel , followed by lyophilization of the solution to obtain paclitaxel micellar lyophilized powder. After this lyophilized powder is redissolved, the measured particle diamet...
Embodiment 3
[0061] Example 3 Fmoc group capping paclitaxel
[0062] Dissolve 1.84g of Fmoc lysine in 10ml of anhydrous ethyl acetate, add 0.7ml of triethylamine, cool the solution to -10°C, add 0.61ml of pivaloyl chloride, stir and raise the temperature to 0°C for 2 hours, then warm up to room temperature and react again After 1h, the insoluble matter was removed by filtration, and the filtrate was rotary evaporated to remove the solvent to obtain a white solid dissolved in 5ml of dry dichloromethane, 0.7ml of triethylamine and 74mg of 4-pyrrolidinylpyridine were added, the solution was cooled to 0°C, and the PLA- 1 g of PEG-PLA dendritic polymer was reacted for 2 hours, and the reaction was continued at room temperature for 24 hours to obtain Fmoc-Lys endcapped mPEG-PLA, an Fmoc-endcapped polymer.
[0063] Dissolve 1 g of the Fmoc-capped polymer obtained in the above steps and an appropriate amount of paclitaxel in ethanol, remove the solvent by rotary evaporation at 45 ° C, add 50 ml of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com