Co-delivery system of photo-responsive chemotherapeutic drug and preparation method thereof
A chemotherapeutic drug and co-delivery technology, applied in the field of medicine, can solve the problems of inability to directly apply multi-drug co-delivery and low delivery efficiency, and achieve the effects of improving curative effect and targeting, good stability, and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] 1. Preparation of double-branched photosensitive carrier:
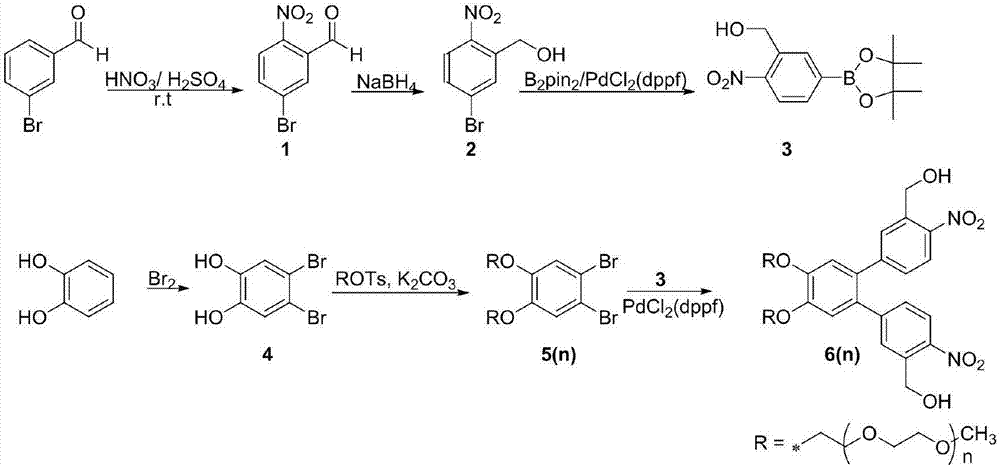
[0052] The method reported in the reference (Tetrahedron Letters, 2016, 57, 959-963), the preparation route is as follows figure 1 As shown, the steps are as follows:
[0053] (1) Take 20mL of concentrated nitric acid, stir in an ice bath to 0°C, add 25mL of concentrated sulfuric acid into the pipette, stir for 20min in an ice bath, and then add 10g of m-bromobenzaldehyde. Stir at room temperature for 4 h, add a small amount of water, and adjust the pH to 3-4 with NaOH. Then it was extracted three times with ethyl acetate, and the organic phase was concentrated by rotary evaporation to obtain product 1; the product 1 was dissolved in 25 mL of methanol, and 2 g of sodium borohydride was added in batches under an ice bath, and stirred at room temperature for 2 h. 20 mL of water was added to the reaction solution, and then extracted three times with 20 mL of ethyl acetate, the organic phases were combined, dried...
preparation Embodiment 1
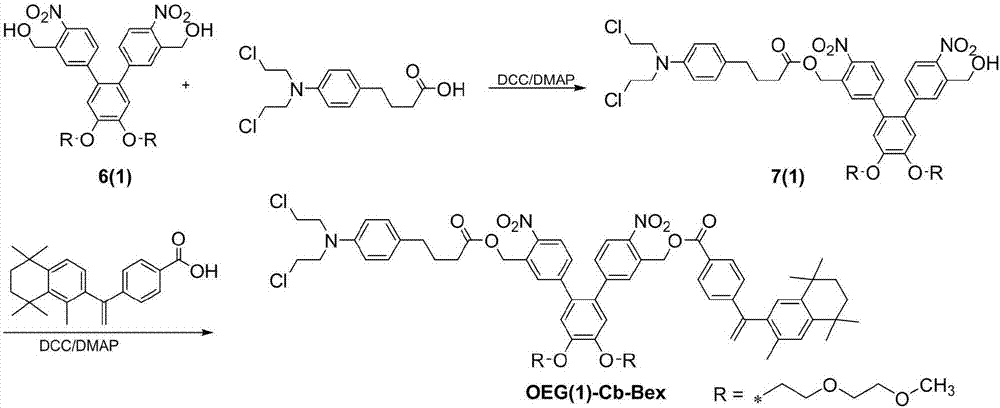
[0060] The preparation route of chlorambucil-bexarotene co-delivery system [OEG(1)-Cb-Bex] is as follows figure 2 shown, including the following steps:
[0061] (1) Weigh 0.4 g of double-branched photosensitive carrier 6 (1) and dissolve it in 15 mL of dichloromethane, add 0.24 g of chlorambucil, 0.2 g of dicyclohexylcarbodiimide and a small amount of 4- Dimethylaminopyridine was reacted with stirring at room temperature for 4 h; 10 mL of water was added, extracted three times with ethyl acetate, the organic phase was concentrated and then purified by column to obtain 0.29 g of yellow product 7(1), with a yield of 50%;
[0062] (2) Weigh 0.2 g of product 7 (1) and dissolve it in 15 mL of dichloromethane, add 0.1 g of bexarotene, 70 mg of dicyclohexylcarbodiimide and a small amount of 4-dimethylaminopyridine, and The reaction was stirred for 4 h; 10 ml of water was added, extracted three times with ethyl acetate, the organic phase was concentrated, and the residue was purifie...
preparation Embodiment 2
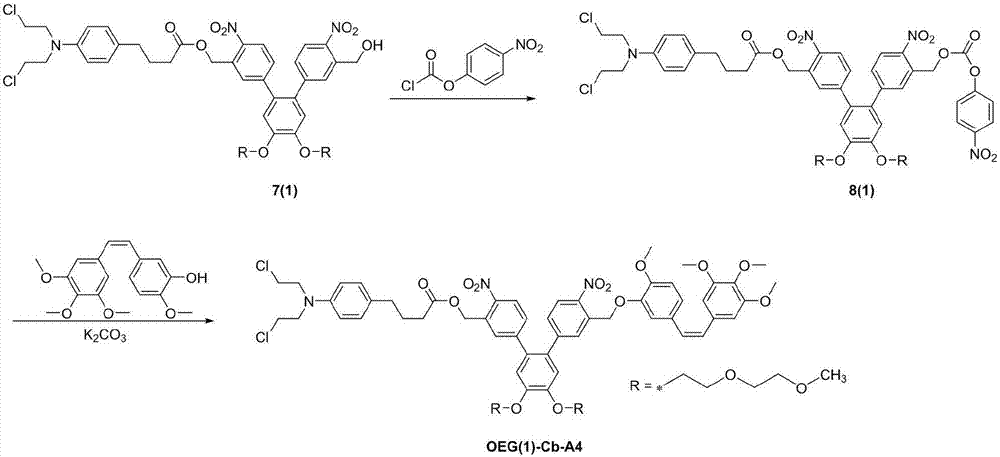
[0064] The preparation route of chlorambucil-compretin A4 co-delivery system [OEG(1)-Cb-A4] is as follows image 3 shown, including the following steps:
[0065] (1) Weigh 0.8 g of double-branched photosensitive carrier 6 (1) and dissolve it in 30 mL of dichloromethane, add 0.48 g of chlorambucil, 0.4 g of dicyclohexylcarbodiimide and a small amount of 4- Dimethylaminopyridine was reacted with stirring at room temperature for 4 h; 20 mL of water was added, extracted three times with ethyl acetate, the organic phase was concentrated and then purified by column to obtain 0.58 g of yellow product 7(1), with a yield of 50%;
[0066] (2) Weigh 0.4 g of the product 7 (1) and dissolve it in 15 mL of chloroform, add 0.13 g of p-nitrophenyl chloroformate and 0.2 mL of triethylamine, and stir at room temperature for 3 h. 20 mL of water was added to the reaction solution, extracted three times with 15 mL of ethyl acetate, the organic phases were combined and concentrated, and the residu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com