Simple preparation method for Fe3O4@C@TiO2 magnetic separation photocatalyst
A photocatalyst and magnetic separation technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical instrument and method, etc., can solve bad heterojunction, electron-hole recombination photodissolution The probability of increased and other issues, to achieve the effect of low equipment requirements, good visible light photocatalytic activity, high-efficiency magnetic recovery characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
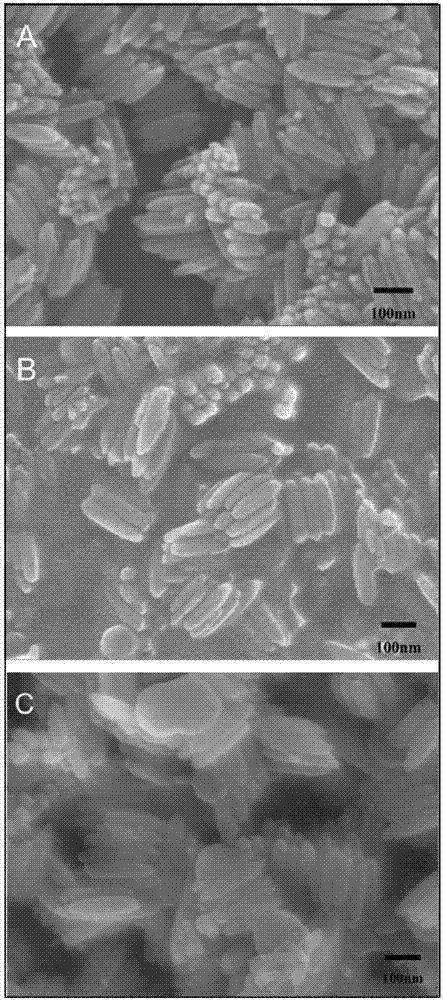
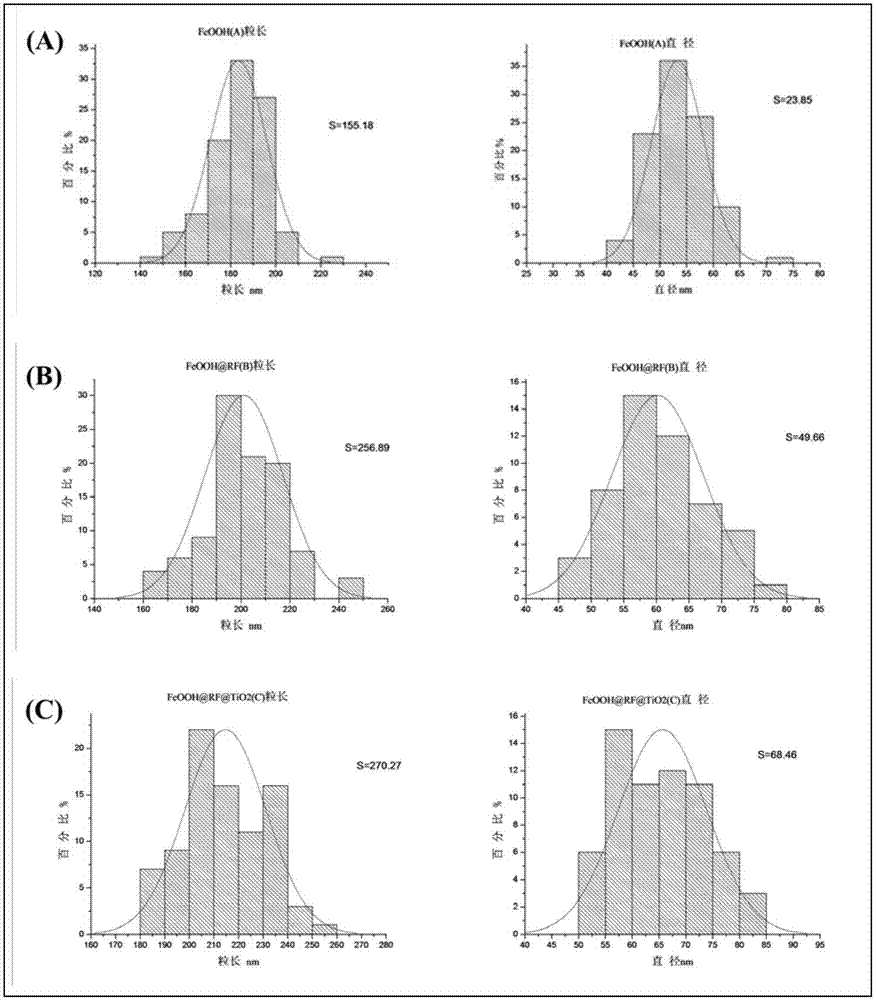
[0032] Magnetically separable Fe 3 o 4 @C@TiO 2 The preparation process of photocatalyst is as follows: 0.1g CTAB and 0.108g FeCl 3 ·6H 2 O (0.4 mmol) was dissolved in 4 mL of water. After fully dissolved, centrifuge for 3 minutes and discard the sediment. The clarified solution was slowly magnetically stirred at 85°C for 12h. centrifuged (1000rpm 3min), washed 3-5 times with water to obtain β-FeOOH nanorods. Then 0.03 g of β-FeOOH nanorods were ultrasonically dispersed in 20 mL of water to form a dispersion. Add 1mL of 0.1M PAA solution to the above 20mL dispersion liquid, stir and disperse at a medium speed for 12h, centrifuge at 1000rpm to recover the solid and redisperse it in 28mL water, add resorcinol (R) solution (0.05g resorcinol dissolved in 1ml water) and 70 μL of formaldehyde (F) solution, stirred for 5 min, added dropwise 1 mL of 28% ammonia water at a constant speed, and reacted for 12 h at room temperature under magnetic stirring. The obtained product was...
Embodiment 2
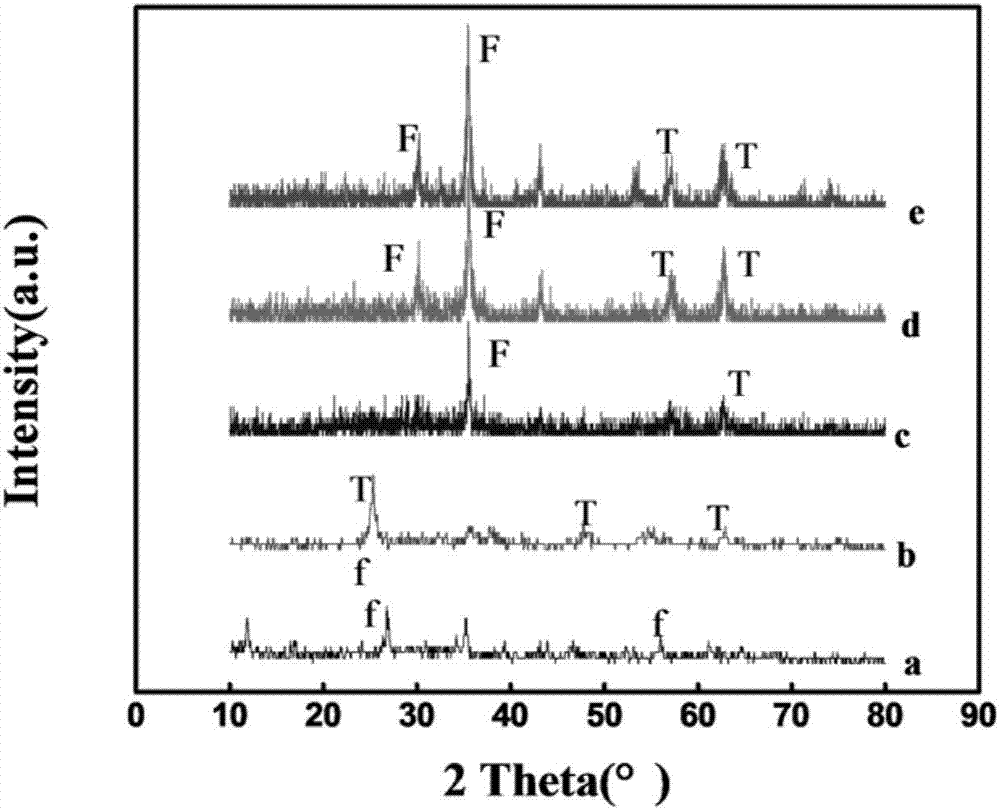
[0042] In order to examine the effect of different calcination temperatures on Fe 3 o 4 @C@TiO 2 The influence of magnetic separation photocatalyst preparation and photocatalytic performance, in addition to calcination temperature, other reaction conditions such as the concentration of FeOOH template dispersion (1.5mg / mL) and calcination gas environment use N 2 Equally identical with embodiment 1, result is as image 3 , 4 c, e and Figure 9 As shown in A and C, at 400°C and 550°C, N 2 The sample Fe obtained by calcination in the atmosphere 3 o 4 @C@TiO 2 Both have significant Fe 3 o 4 、TiO 2 The characteristic diffraction peaks, and their UV-Vis diffuse reflectance spectra have strong absorption peaks in the range of 200-800nm. Calcined at 400°C Figure 9 After photocatalytic degradation of A, the peak value in the entire ultraviolet-visible range decreased slightly, indicating that a small amount of methyl orange was degraded. Figure 9 C shows that the characte...
Embodiment 3
[0045] In order to examine the effect of the calcination gas environment on Fe 3 o 4 @C@TiO 2 The influence of magnetic separation photocatalyst preparation and performance, except the gas environment of calcination, other reaction conditions such as the concentration (1.5mg / mL) of FeOOH template dispersion liquid and calcination temperature etc. are all identical with embodiment 1, the result is as follows image 3 , 4 Shown in b. Fe was not observed when the calcination was carried out in a non-inert gas 3 o 4 The characteristic diffraction peaks of 2 Can't get Fe 3 o 4 @C@TiO 2 , and FeOOH can be successfully reduced to Fe under nitrogen atmosphere 3 o 4 , and b is similar to a, with a strong absorption peak in the range of 200-400nm, but a weak absorption peak in the range of 400-800nm, indicating that b also only responds to ultraviolet light and has no response to visible light.
[0046] Calcination gas environment on Fe 3 o 4 @C@TiO 2 Magnetic separation T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com