Polypeptide liquid-phase synthesizing method of oxytocin
A technology of oxytocin and polypeptide liquid, which is applied in the field of polypeptide liquid phase synthesis, can solve the problems of difficult to control reaction conditions, low yield and titer of oxytocin products, and achieves low synthesis cost, high titer and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Synthetic Fragment 1: Boc-Cys(Acm)-Tyr(tBu)-OH
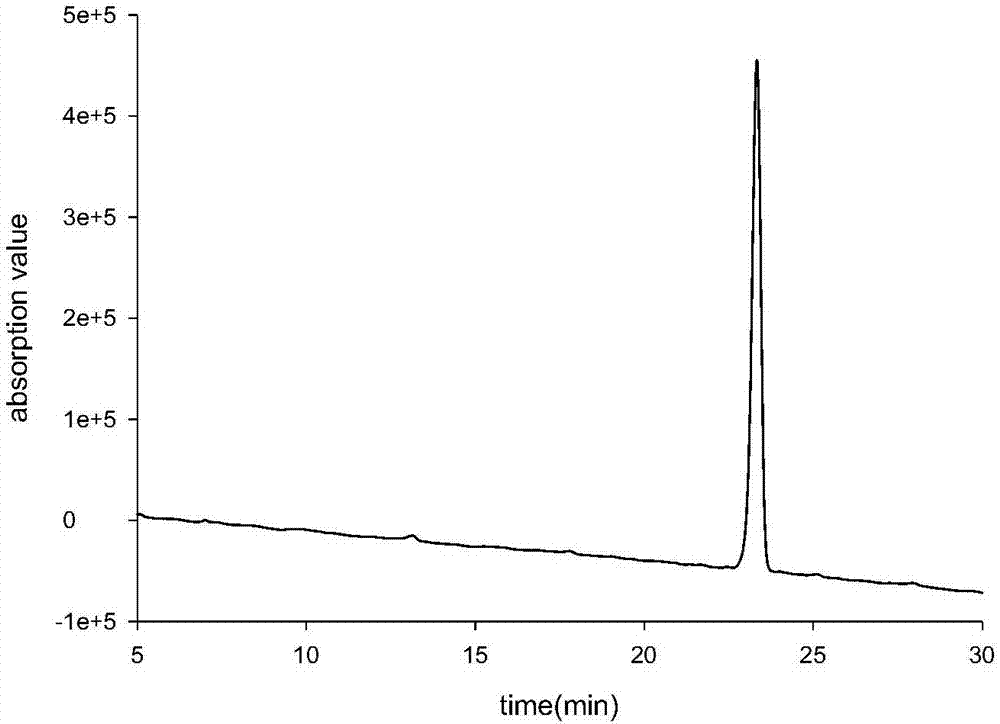
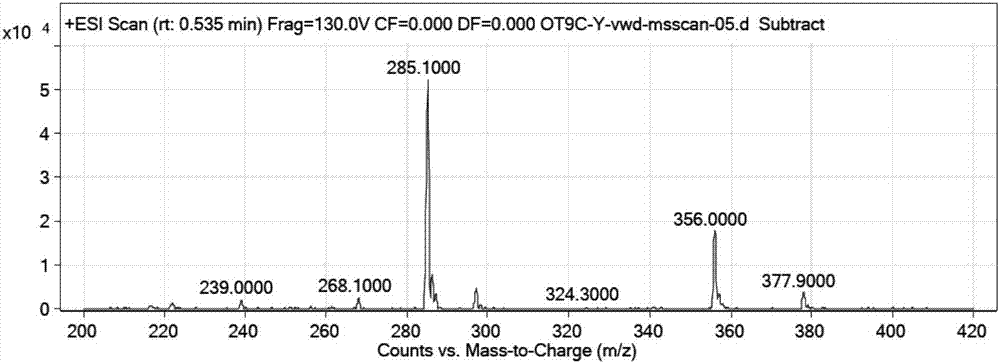
[0084] Dissolve Boc-Cys(Acm)-OH (100mol, 29.2g, 1eq.), HOSu (110mmol, 12.6g, 1.1eq.) in 200ml of tetrahydrofuran, cool at -10°C for 10min, DCC (110mmol, 22.7g, 1.1eq.) was dissolved in 20ml THF, added to the above solution, reacted for 20min, weighed H-Tyr(tBu)-OH (110mmol, 26.1g, 1.1eq.) and NaHCO 3 (110mmol, 9.2g, 1.1eq.) was dissolved in 150ml of water and added to the above solution. React at 15-25°C, monitor the reaction by HPLC, adjust the pH to neutral with 0.5M hydrochloric acid, filter out the insoluble matter, concentrate to remove THF, adjust the pH to 2 with 0.5M hydrochloric acid to obtain a white solid, filter, wash with water until neutral, and dry in vacuo Fragment 1 was obtained with a purity of 99.1%, 47.1 g of the product, and a yield of 92%. Fragment 1: Boc-Cys(Acm)-Tyr(tBu)-OH RP-HPLC spectrum see attached figure 1 , its high acid cleavage sample (H-Cys(Acm)-Tyr-OH m / z calculated.355.4; found 356.3...
Embodiment 2
[0086] Synthetic Fragment 2: H-Ile-Gln(Trt)-Asn(Trt)-Cys(Acm)-Pro-OMe
[0087] (1) Synthesis of Compound 2.1: Fmoc-Cys(Acm)-Pro-OMe:
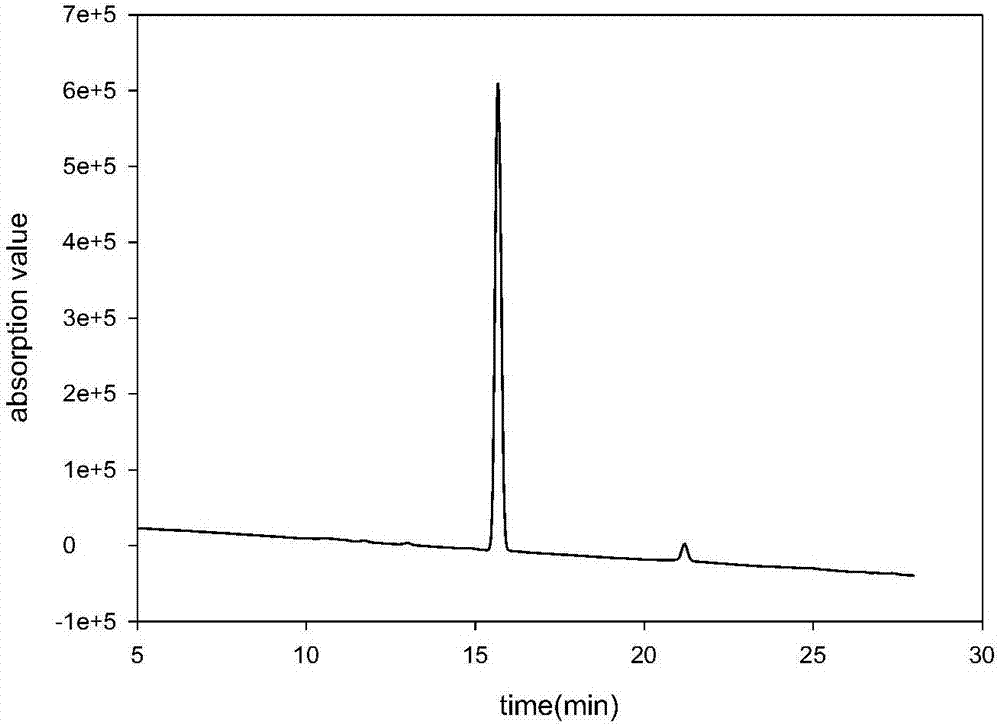
[0088] Dissolve Fmoc-Cys(Acm)-OH (100mmol, 41.5g, 1eq.), HOBt (105mmol, 14.1g, 1.05eq.) in 200ml THF and 30ml DMF, cool at -10°C for 10min, DCC (105mmol, 21.6g, 1.05eq.) was dissolved in 20ml THF, added to the above solution, and after reacting for 20min, weigh H-Pro-OMe. , 1.05eq.), mix well and add to the above reaction solution. Reaction at 15-25°C, HPLC monitoring for complete reaction, filtration to remove insoluble matter, concentration, precipitation of solid with 0.1M hydrochloric acid, filtration, washing to neutrality, vacuum drying to obtain compound 2.1, purity 95.3%, product 50.9g, yield 97 % (yields are all calculated on the basis of 100 mmol, the same below). See the attached manual for the HPLC analysis chart image 3 .
[0089] (2) Synthesis of Compound 2.2: H-Cys(Acm)-Pro-OMe:
[0090] The dried compound 2.1 was dissolve...
Embodiment 3
[0104] Synthesis of Fragment 3: H-Leu-Gly-NH according to Scheme 2 2
[0105] Weigh Fmoc-Leu-OH (1eq., 100mmol, 35.3g), BOP (1.01eq., 101mmol, 44.7g) dissolved in DMF200ml, add DIPEA (1.2eq., 120mmol, 21ml), after 5min, called H-Gly -NH 2 .HCl (1.1eq., 110mmol, 12.2g) was dissolved in 100ml of DMF, added TEA (1.1eq., 110mmol, 15ml), poured into the above reaction solution, followed by TLC (DCM:MeOH:AcOH=100:6:1) After monitoring, after the reaction is complete, precipitate with 0.1M HCl solution, wash with water until neutral, and dry in vacuum to obtain the product Fmoc-Leu-Gly-NH 2 .HCl, purity 97%, product 43.7g, yield 98%, HPLC analysis spectrum see attached Figure 10 .
[0106] Dry Fmoc-Leu-Gly-NH 2 Add 400ml of diethylamine to HCl, track and monitor with TLC (DCM:MeOH:AcOH=100:6:1), after the reaction is complete, concentrate, precipitate petroleum ether, filter, wash with petroleum ether, and dry in vacuo to obtain Fragment 3 with a purity of 98.4% , product 17.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com