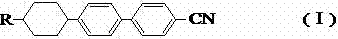
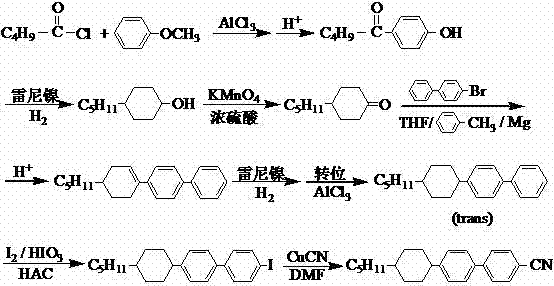
Preparation method of alkyl cyclohexyl cyanobiphenyl liquid crystal compound
A technology based on liquid crystal compounds and biphenylnitrile, which is applied in chemical instruments and methods, liquid crystal materials, etc., and can solve problems such as high safety risks, environmental pollution, and unreasonable synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Taking the preparation of n-pentylcyclohexylbiphenylnitrile {trans-4-(4-n-pentylcyclohexyl)-[1,1-biphenyl]-4-carbonitrile)} liquid crystal compound (I-1) as an example , specify the preparation method of pentylcyclohexyl biphenyl nitrile liquid crystal compound:
[0073] Step a: Preparation of trans-4-(4-n-pentylcyclohexyl)phenylboronic acid (Ⅲ-1):
[0074] Install equipment and feed: Add 14L dry tetrahydrofuran to the reaction kettle, install the reaction kettle, nitrogen protection device (balloon), dropping device and cooling device, vent nitrogen for 3 to 4 times, maintain a slight positive pressure, and maintain It takes about 1 to 1.5 hours for the balloon to stand in a stable state, pass liquid nitrogen to cool down, control the cooling speed, and prevent the balloon from sucking back.
[0075] Cooling+dropping 1: When the temperature drops below -80°C, use a nitrogen pressure device to press 11mol of n-butyllithium into the dropping funnel at a stable speed, th...
Embodiment 2
[0104] Preparation of trans-4-(4-n-pentylcyclohexyl)phenylboronic acid (Ⅲ-1):
[0105] The preparation process is the same as in Example 1, except that the n-butyllithium in step a is replaced by sec-butyllithium, and the other steps are similar, and 2.49kg of white solid---HPLC≥99.0% of the target product (Ⅲ -1), yield: 91%.
Embodiment 3
[0107] Preparation of trans-4-(4-n-pentylcyclohexyl)phenylboronic acid (Ⅲ-1):
[0108] The preparation process is the same as in Example 1, except that the 2.76kg triisobutyl borate raw material in step a is replaced by 1.250kg trimethyl borate, and other steps are similar, and 2.44kg white solid is prepared---HPLC≥99.0% The target product (Ⅲ-1), yield: 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com