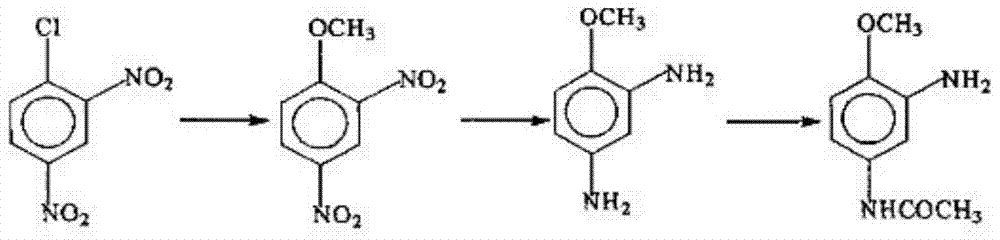
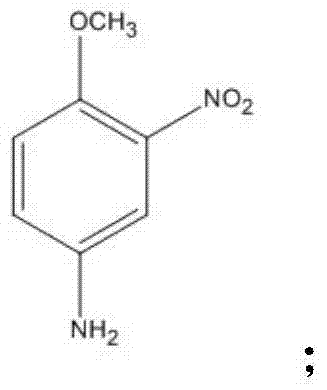
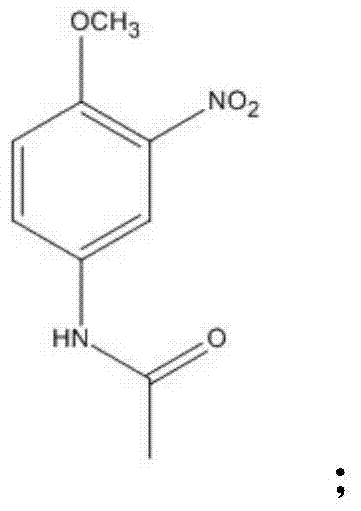
Disperse dye intermediate preparation method
A technology for disperse dyes and intermediates, applied in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problems of rising industrial production costs, reduced yields, metal pollution, etc. The effect of less impurities and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment one A, synthetic route A
[0064] (1) Add 1700g m-phenylenedinitro, 2000g methanol (the amount of the solvent can also be 3200g, 4000g, 5000g, 8000g), 600-1200g strong acid ion exchange resin, 3% Pt in the pressure reactor -C catalyst 12g (or 15g, 20g, 45g, etc.), after nitrogen protection, replace hydrogen with nitrogen, and react at a temperature of 50°C with a hydrogen pressure of 0.02MPa (0.1MPa, 0.5MPa, 2MPa) for 5 hours ( 1-10 hours); filter to remove insoluble matter, distill methanol and place it for later use. (Ion exchange resin removed by filtration can be recycled)
[0065] (2) After cooling, add 1000g of acetic anhydride (the amount of acetic anhydride can also be 2000g, 2500g, 3000g, 4000g), reflux reaction for 2 hours (ranging from 1-8 hours), heat filtration to remove solid residue, distill When there is no distillate, keep it for use.
[0066] (3) Add the above product and 3% Pt-C catalyst (or 15g, 20g, 45g) in the pressure reactor, after ...
Embodiment 1
[0071] Embodiment one B, synthetic route B
[0072] On the basis of the above-mentioned reaction process, in step (1), add 1700g m-dinitrobenzene, zinc powder 250g (or be 300g, 500g, 1000g not etc., or replace iron and aluminium) as in the pressure reactor, Hydroxylamine is obtained under the action of 1200g98% sulfuric acid (or 2300g, 3500g, 4000g), 1000g water (or 5000g, 10000g, 20000g) and carbon dioxide pressure of 0.02MPa (0.1MPa, 0.5MPa, 2MPa). After the product, the insoluble matter was removed by filtration;
[0073] Add 1200g of 98% acetic acid (or 2300g, 3500g, 4000g), 2000g methanol (the amount of the solvent can also be 3200g, 4000g, 5000g, 8000g) to react to obtain amine in the reactor of the above reaction intermediate products.
[0074] It is worth noting that in the above two types of embodiments, when aluminum, zinc or iron is used as the reducing agent, after the reaction, the pH of the reaction solution is adjusted to precipitate metal ions, and after filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com