Method for preparing 5-formyloxy methylfurfural from fructose
A technology of formyloxymethylfurfural and fructose, applied in the direction of organic chemistry, etc., can solve the problems of low yield and small feeding amount, and achieve the effects of low preparation cost, high feeding amount and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
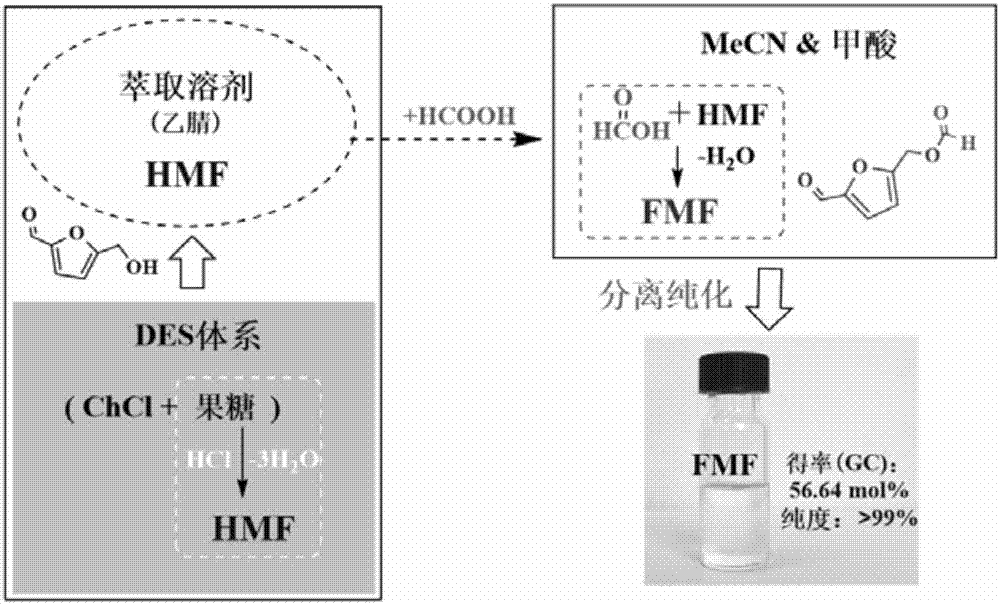
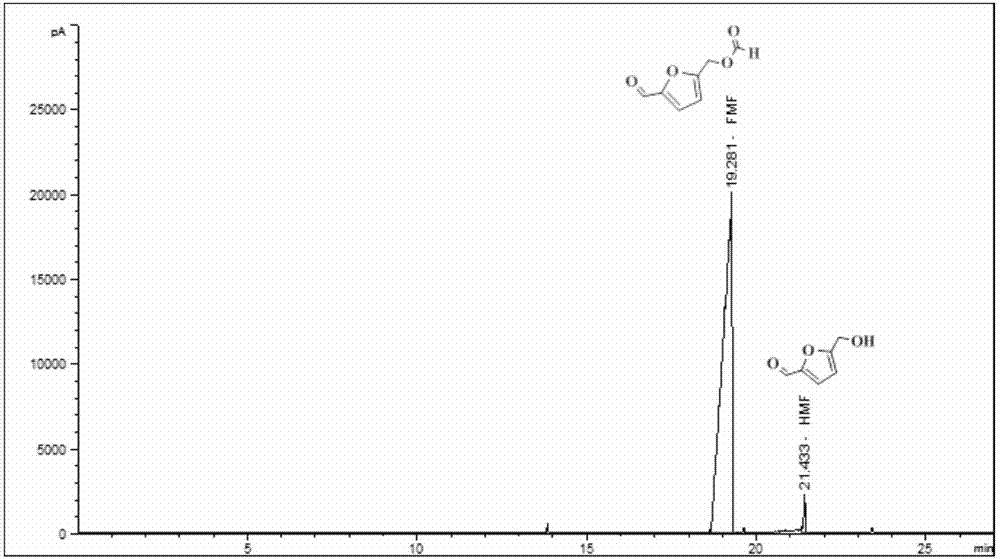
[0026] Add 1.08g of choline chloride and 0.27g of fructose into a 50mL round bottom flask, shake and mix properly. Another 10 mL of tetrahydrofuran and 2 μL of hydrochloric acid (38%) were added. The flask was placed in an oil bath for magnetic stirring, and the reaction was heated at a temperature of 100° C., and the reaction time was 4 hours. After the reaction was completed, it was cooled naturally. Take out the upper reaction solution, add anhydrous formic acid (anhydrous formic acid:reaction solution volume ratio is 1:2), heat the reaction in an oil bath, the temperature is 100°C, and react for 6h. After the reaction, the reaction solvent was distilled off under reduced pressure, the residue was extracted several times with 5 mL of methyl tert-butyl ether, the extracts were combined, and the extractant was recovered by distillation under reduced pressure. Rectify at 125-130°C under a pressure of 2000 Pa, wash the distillate with saturated aqueous sodium bicarbonate solu...
Embodiment 2
[0028] Add 1.08g of choline chloride and 0.27g of fructose into a 50mL round bottom flask, shake and mix properly. Another 10 mL of acetonitrile and 2 μL of hydrochloric acid (38%) were added. The flask was placed in an oil bath for magnetic stirring, and the reaction was heated at a temperature of 100° C., and the reaction time was 4 hours. After the reaction was completed, it was cooled naturally. Take out the upper reaction solution, add anhydrous formic acid (anhydrous formic acid:reaction solution volume ratio is 1:2), heat the reaction in an oil bath, the temperature is 100°C, and react for 6h. After the reaction, the reaction solvent was distilled off under reduced pressure, the residue was extracted several times with 5 mL of methyl tert-butyl ether, the extracts were combined, and the extractant was recovered by distillation under reduced pressure. Rectify at 125-130°C under a pressure of 2000 Pa, wash the distillate with saturated aqueous sodium bicarbonate solutio...
Embodiment 3
[0030] Add 1.08g of choline chloride and 0.27g of fructose into a 50mL round bottom flask, shake and mix properly. Another 20 mL of methyl isobutyl ketone and 2 μL of hydrochloric acid (38%) were added. The flask was placed in an oil bath for magnetic stirring, and the reaction was heated at a temperature of 100° C., and the reaction time was 4 hours. After the reaction was completed, it was cooled naturally. Take out the upper reaction solution, add anhydrous formic acid (anhydrous formic acid:reaction solution volume ratio is 1:4), heat the reaction in an oil bath, the temperature is 80°C, and react for 6h. After the reaction was over, the reaction solvent was distilled off under reduced pressure, and the residue was extracted several times with a mixture of 5 mL of ethyl acetate and n-hexane (volume ratio 1:1) continuously, the extracts were combined, and the extractant was recovered by distillation under reduced pressure. Rectify at 125-130°C under a pressure of 2000 Pa,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com