Tumor specifically-enriched nanometer drug delivery system and preparation method thereof
An active and drug-based technology, applied in the field of amphiphilic block copolymers, can solve the problem of insufficient specific enrichment of drug transport and achieve high drug loading efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
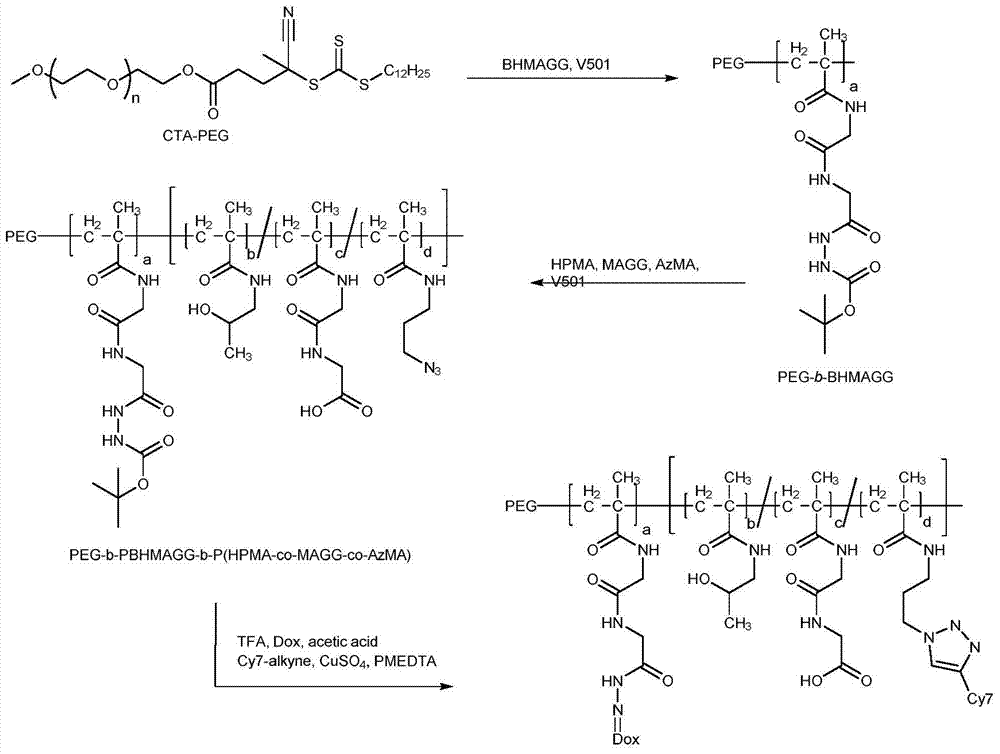
[0161] PEG 5k -b-PBHMAGG 9k -b-P(HPMA-co-MAGG-co-AzMA) 60k -SC(=S)S(CH 2 ) 11 CH 3 preparation of
[0162] Synthetic route such as figure 1 shown.
[0163] (1) Dissolve 10 g of hydroxyl and methyl-terminated PEG with a number average molecular weight of 5000 in 40 mL of toluene, and after azeotropic water removal twice with toluene, add 4-dodecyltrithiocarbonate-4-carbonitrile 3.23 g of valeric acid and 0.1 g of DMAP were dissolved in 70 ml of toluene, and after complete dissolution, 1.98 g of DCC was added and reacted for 90 hours under stirring at room temperature. After the reaction, remove cyclohexyl urea (DCU) by suction filtration, then pour the filtrate into excess ether, collect the precipitate, dissolve the obtained precipitate in a small amount of toluene, and then precipitate with ether, repeat this 3-5 times until the ether is colorless. The precipitate was vacuum-dried at 40° C. for 24 hours, and the obtained product was a macromolecular chain transfer age...
Embodiment 2
[0168] PEG 5k -b-PBHMAGG 9k -b-P(HPMA-co-MAGG-co-AzMA) 30k -SC(=S)S(CH 2 ) 11 CH 3 preparation of
[0169] Using the same reaction route and reaction conditions as in Example 1, the addition of HPMA, MAGG and AzMA was adjusted to 0.18g, 0.02g and 0.005g, and the title final product was prepared by polymerization.
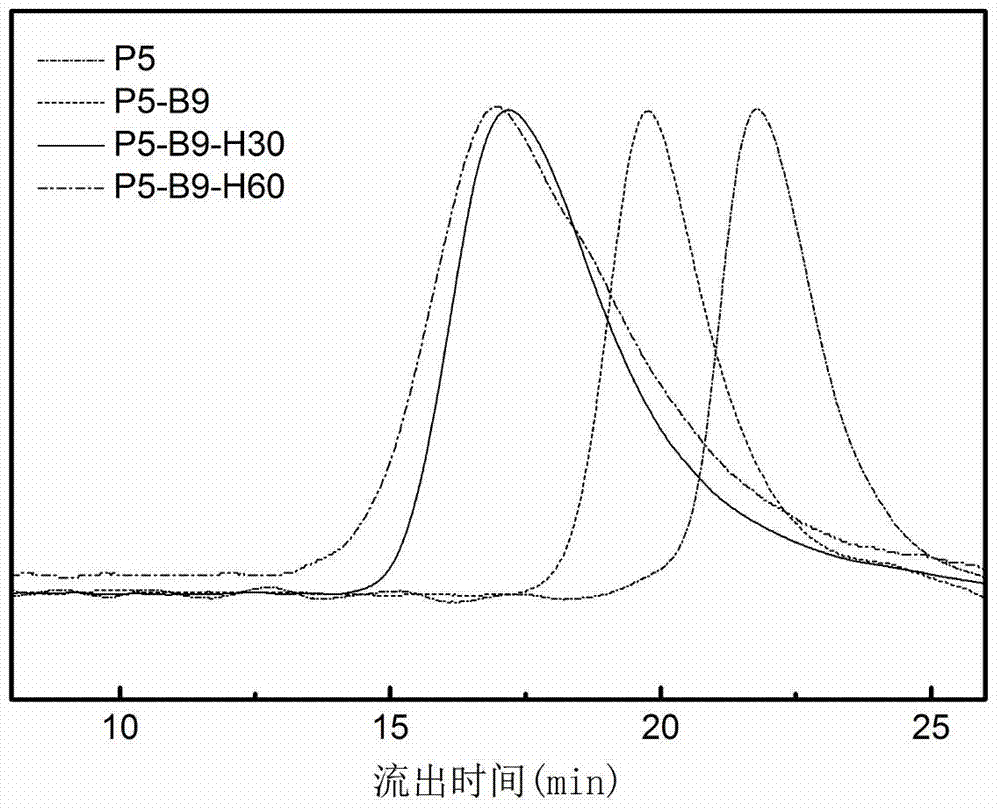
[0170] Hereinafter, this polymer is simply referred to as P5-B9-H30.
Embodiment 3
[0172] PEG 5k -b-PBHMAGG 5k -b-P(HPMA-co-MAGG-co-AzMA) 60k -SC(=S)S(CH 2 ) 11 CH 3 preparation of
[0173] Using the same reaction route and reaction conditions as in Example 1, the amount of BHMAGG added was adjusted to 0.35 g, and the title final product was prepared by polymerization.
[0174] Hereinafter, this polymer is simply referred to as P5-B5-H60.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com