Method for preparing isoproterenol hydrochloride
A technology of isoproterenol hydrochloride and epinephrine, applied in the field of medicine, can solve the problems of difficult treatment of phosphorus-containing wastewater, great harm to human body, low reaction yield, etc., achieve obvious economic and environmental benefits, and facilitate industrial production , the effect of less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
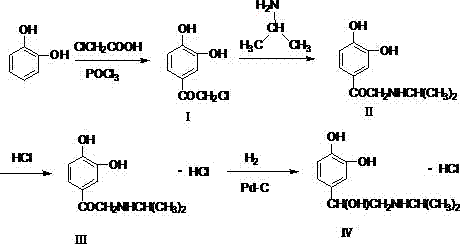
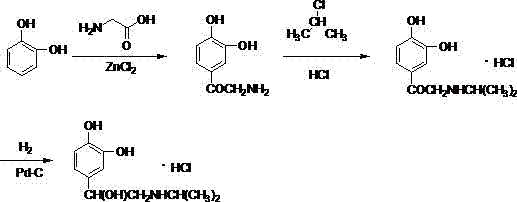
[0031] (1) Preparation of 2-amino-1-(3,4-dihydroxyphenyl)-ethanone:
[0032] Add 1L of 1,2-dichloroethane into the reaction bottle, add 330g of zinc chloride when the temperature is lowered to 10-15°C, stir for 20 minutes after the addition, add catechol 110g (1.0mol) in batches to the reaction In the bottle, continue to stir for 30 minutes after the addition, then raise the temperature to 70°C, add dropwise a solution of 75g (1.0mol) of glycine in 1,2-dichloroethane, heat up to reflux for 12 hours after the addition, and cool down after the reaction to room temperature, add dilute hydrochloric acid to quench, stir at 20-30°C for 2-3 hours, collect the solid by filtration, adjust the pH of the solid to about 6.7 with aqueous sodium bicarbonate solution, filter, collect the solid, and dry to obtain the product 2- Amino-1-(3,4-dihydroxyphenyl)-ethanone 125.3 g, yield 75.02%.
[0033] (2) Preparation of isoproterenol ketone body hydrochloride
[0034] Add 250g of 95% ethanol an...
Embodiment 2
[0038] (1) Preparation of 2-amino-1-(3,4-dihydroxyphenyl)-ethanone:
[0039] Add 1L of 1,2-dichloroethane into the reaction bottle, add 450g of zinc chloride when the temperature is lowered to 10-15°C, stir for 20 minutes after the addition, add catechol 110g (1.0mol) in batches to the reaction In the bottle, continue to stir for 30 minutes after the addition, then raise the temperature to 70°C, add dropwise a solution of 78.6g (1.05mol) of glycine in 1,2-dichloroethane, and raise the temperature to reflux for 20 hours after the completion of the reaction. Cool down to room temperature, add dilute hydrochloric acid to quench, stir at 20-30°C for 2-3 hours, collect the solid by filtration, adjust the pH of the solid to about 6.8 with aqueous sodium bicarbonate solution, filter, collect the solid, and dry to obtain product 2 -Amino-1-(3,4-dihydroxyphenyl)-ethanone 128.3 g, yield 76.82%.
[0040] (2) Preparation of isoproterenol ketone body hydrochloride
[0041] Add 250g of 95...
Embodiment 3
[0045] (1) Preparation of 2-amino-1-(3,4-dihydroxyphenyl)-ethanone:
[0046] Add 1L of 1,2-dichloroethane into the reaction bottle, add 550g of zinc chloride when the temperature is lowered to 10-15°C, stir for 20 minutes after the addition, add catechol 110g (1.0mol) in batches to the reaction In the bottle, continue to stir for 30 minutes after the addition, then raise the temperature to 70°C, add dropwise a solution of 82.5g (1.1mol) of glycine in 1,2-dichloroethane, after the addition, raise the temperature to reflux for 20 hours, after the reaction Cool down to room temperature, add dilute hydrochloric acid to quench, stir at 20-30°C for 2-3 hours, and collect the solid by filtration. Adjust the pH of the solid to about 7 with aqueous sodium bicarbonate solution, filter, collect the solid, and dry to obtain 131.6 g of the product 2-amino-1-(3,4-dihydroxyphenyl)-ethanone with a yield of 78.8% .
[0047] (2) Preparation of isoproterenol ketone body hydrochloride
[0048]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com