Myricetin derivative containing piperazine amides and preparation method thereof
A technology of myricetin and amides, which is applied to the preparation of myricetin derivatives containing piperazine amides, and the application field of anti-tumor, can solve the problems of less research on the anti-tumor activity of myricetin derivatives, and achieve toxic and side effects. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
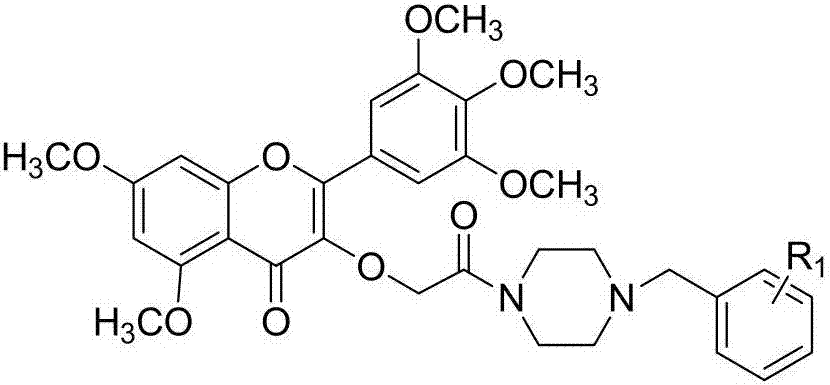
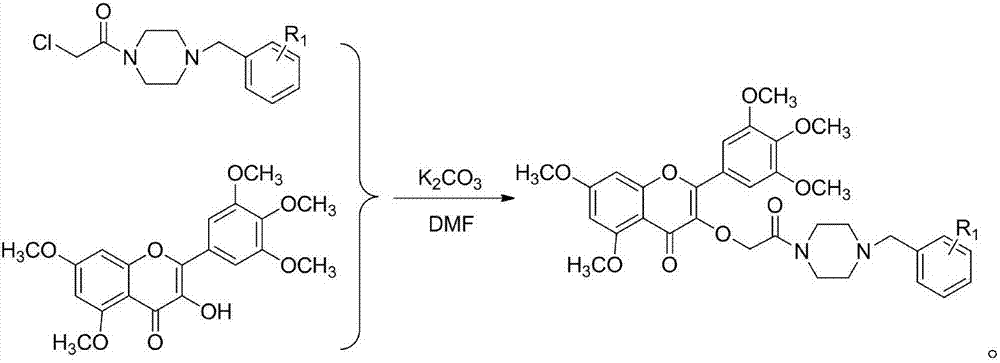
[0041] 3-(2-(4(2-chlorophenyl)piperazinyl)-2-oxoethyl)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl )-4H-chromene-4-ketone synthesis (compound number is I 1 ), including the following steps:
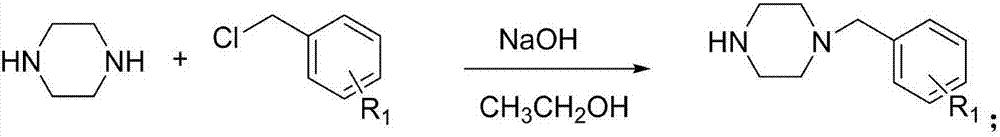
[0042] (1) Synthesis of 1-(2-chlorobenzyl) piperazine:
[0043] Add 2.59g (30mmol) of anhydrous piperazine, 1.20g (30mmol) of sodium hydroxide and 25mL of absolute ethanol to a 50mL single-necked flask, stir at room temperature until it is completely dissolved, add 1.27g (10mmol) of substituted benzyl chloride, and Raise the temperature to reflux until the benzyl chloride completely disappears for about 5-7 hours. Stop the reaction, remove the solvent under reduced pressure, add water to dissolve, adjust pH>12, extract with dichloromethane (3×30mL), combine the organic layers and dry over anhydrous sodium sulfate, filter with suction, remove the solvent under reduced pressure to obtain the crude product without Purification was used directly in the next step.
[0044] (2) Synthesis of 1-(...
Embodiment 2
[0051] 3-(2-(4(2,4-dichlorophenyl)piperazinyl)-2-oxoethyl)-5,7-dimethoxy-2-(3,4,5-trimethoxy phenyl)-4H-chromen-4-ketone synthesis (compound number is I 2 ), including the following steps:
[0052] (1) Synthesis of 1-(2,4-dichlorobenzyl)piperazine:
[0053] As in step (1) of Example 1, the difference is that 2,4-dichlorobenzyl chloride is used as a raw material.
[0054] (2) Synthesis of 1-(4-(2,4-dichlorobenzyl)piperazine)-2-chloroethane-1-one:
[0055] As in step (2) of Example 1, the difference is that 1-(2,4-dichlorobenzyl)piperazine is used as a raw material.
[0056] (3) Synthesis of 3-hydroxy-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one:
[0057] As in embodiment 1 (3) step.
[0058] (4) 3-(2-(4(2,4-dichlorophenyl)piperazinyl)-2-oxoethyl)-5,7-dimethoxy-2-(3,4,5 Synthesis of -trimethoxyphenyl)-4H-chromen-4-one:
[0059] As in step (4) of Example 1, the difference is that 1-(4-(2,4-dichlorobenzyl)piperazine)-2-chloroethane-1-one is used as the raw mate...
Embodiment 3
[0061] 3-(2-(4(3-methylphenyl)piperazinyl)-2-oxoethyl)-5,7-dimethoxy-2-(3,4,5-trimethoxybenzene base)-4H-chromen-4-ketone synthesis (compound number is I 3 ), including the following steps:
[0062] (1) Synthesis of 1-(3-methylbenzyl)piperazine:
[0063] As in step (1) of Example 1, the difference is that 3-methylbenzyl chloride is used as a raw material.
[0064] (2) Synthesis of 1-(4-(3-methylbenzyl)piperazine)-2-chloroethane-1-one:
[0065] As in step (2) of Example 1, the difference is that 1-(3-methylbenzyl)piperazine is used as a raw material.
[0066] (3) Synthesis of 3-hydroxy-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one:
[0067] As in embodiment 1 (3) step.
[0068](4) 3-(2-(4(3-methylphenyl)piperazinyl)-2-oxoethyl)-5,7-dimethoxy-2-(3,4,5-trimethyl Synthesis of oxyphenyl)-4H-chromen-4-one:
[0069] As in step (4) of Example 1, the difference is that 1-(4-(3-methylbenzyl)piperazine)-2-chloroethane-1-one is used as a raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com