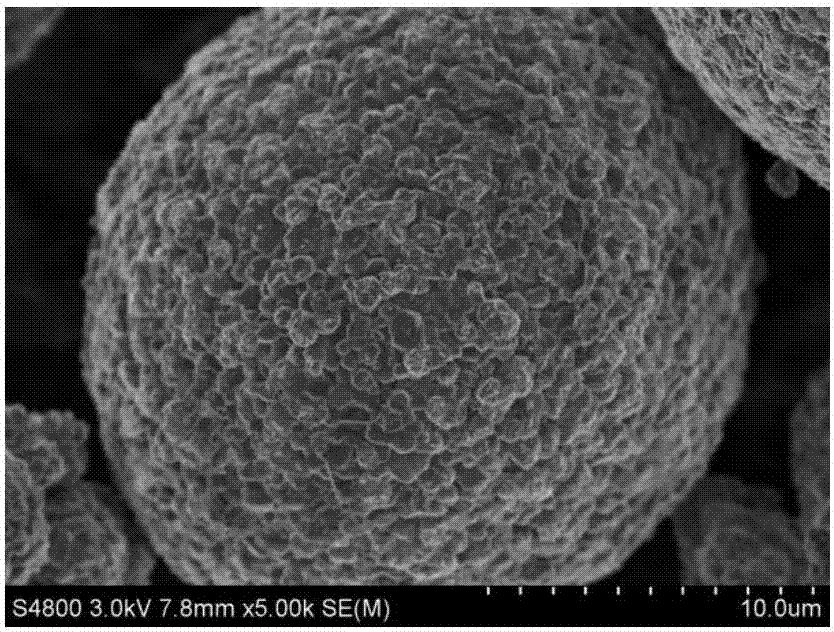
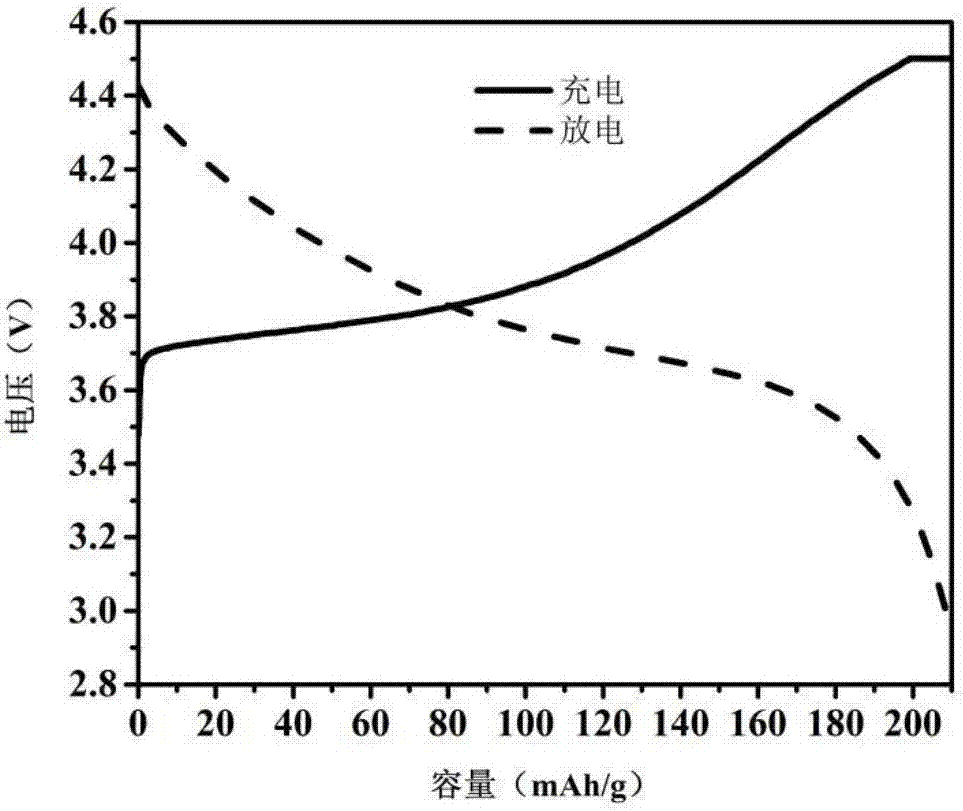
Preparation methods of lithium ion battery composite positive electrode, flexible lithium battery and solid-state lithium battery
A technology for lithium ion batteries and composite positive electrodes, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of low lithium-conducting activity of the coating layer, unfavorable lithium ion transmission, etc., and achieve low cost, improved cycle performance, and preparation. simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Li 7 La 3 Zr 2 o 12 coated LiNi 0.5 mn 1.5 o 4 Positive electrode for all-solid-state batteries
[0026] Step (1) Add 0.57g of lithium nitrate, 1.54g of lanthanum nitrate, 0.55g of zirconium nitrate, 0.8g of ethylene glycol, and 3g of citric acid into a 25ml beaker, and stir at room temperature for 5 hours. According to the mass ratio of inorganic fast lithium ion conductor and unmodified positive electrode material is 1:200, the LiNi of 20g 0.5 mn 1.5 o 4 The powder is added to the above-mentioned inorganic fast lithium ion conductor Li 7 La 3 Zr 2 o 12 Mix the two evenly at room temperature, and sonicate in an ultrasonic disperser for 1h, repeat the ultrasonic step and the stirring step alternately, ultrasonic for 10min, and stir for 10min to ensure the mixing uniformity and Dispersion among materials.
[0027] Step (2) The mixed solution in step (1) was placed at 60° C. and stirred for 5 h. With the volatilization of the solvent in the mixed...
Embodiment 2
[0030] Example 2: Li 7 La 3 Zr 0.15 mn 0.05 o 12 coated LiNi 0.5 mn 0.3 co 0.2 o 2 Positive electrode for all-solid-state batteries
[0031] Step (1) lithium carbonate, lanthanum oxide, zirconium oxynitrate, manganese nitrate with Li 7 La 3 Zr 0.15 mn 0.05 o 12 The stoichiometric ratio (5% excess lithium carbonate) pre-prepared 0.2g of Li 7 La 3 Zr 0.15 mn 0.05 o 12 . Add the above several raw materials into a 25ml beaker, add 2g of citric acid, 5mL of ethylene glycol, and 10mL of dilute nitric acid into the beaker and stir at 50°C for 5 hours. In above-mentioned solution, add according to the mass ratio of inorganic fast lithium ion conductor and unmodified cathode material be 1:100, the LiNi of 10g 0.5 mn 0.3 co 0.2 o 2 The powder is added to the above-mentioned inorganic fast lithium ion conductor Li 7 La 3 Zr 0.15 mn 0.05 o 12 Mix the two evenly at room temperature, and sonicate in an ultrasonic disperser for 1h, repeat the ultrasonic step and th...
Embodiment 3
[0035] Example 3: Li 6.7 al 0.1 La 3 Zr 2 o 12 coated LiNi 0.5 mn 0.3 co 0.2 o 2 Positive electrode for liquid battery
[0036] Step (1) lithium carbonate, lanthanum oxide, zirconium oxynitrate, aluminum nitrate with Li 6.7 al 0.1 La 3 Zr 2 o 12 The stoichiometric ratio (5% excess of lithium carbonate) pre-prepared 0.08g of Li 6.7 al 0.1 La 3 Zr 2 o 12 . Add the above several raw materials into a 25ml beaker, add 2g of citric acid, 5mL of ethylene glycol, and 10mL of dilute nitric acid into the beaker and stir at 40°C for 5 hours. According to the mass ratio of the inorganic fast lithium ion conductor to the unmodified positive electrode material is 1:100, 10g of LiNi 0.5 mn 0.3 co 0.2 o 2 The powder is added to the above-mentioned inorganic fast lithium ion conductor Li 6.7 al 0.1 La 3 Zr 2 o 12 Mix the two evenly at room temperature, and sonicate in an ultrasonic disperser for 1h, repeat the ultrasonic step and the stirring step alternately, ultras...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com