Preparation of red fluorescent carbon nanodots, and method used for detecting ferric ions and ascorbic acid
A carbon nanodot, red fluorescence technology, applied in fluorescence/phosphorescence, chemical instruments and methods, measurement devices, etc., can solve problems such as detection limitations, and achieve the effect of simple and easy test method, high sensitivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0042] The carbon quantum dots used in the present invention are prepared by the following steps:
[0043] (1) Add 0.58g of citric acid, 0.76g of thiourea and 10mL of acetone into a 20mL reactor, and react in an oven at 160°C for 8 hours.
[0044] (2) Take out the reacted solution and place it in a centrifuge tube, set the rotation speed at 9000 rpm, and centrifuge for 10 minutes to remove the insoluble matter from the reaction residue.
[0045] (3) An eluent with a volume ratio of petroleum ether and ethyl acetate of 3:1 was prepared to elute the obtained carbon nanodots, and the lower layer of precipitate was removed, and dried in an oven at 60°C.
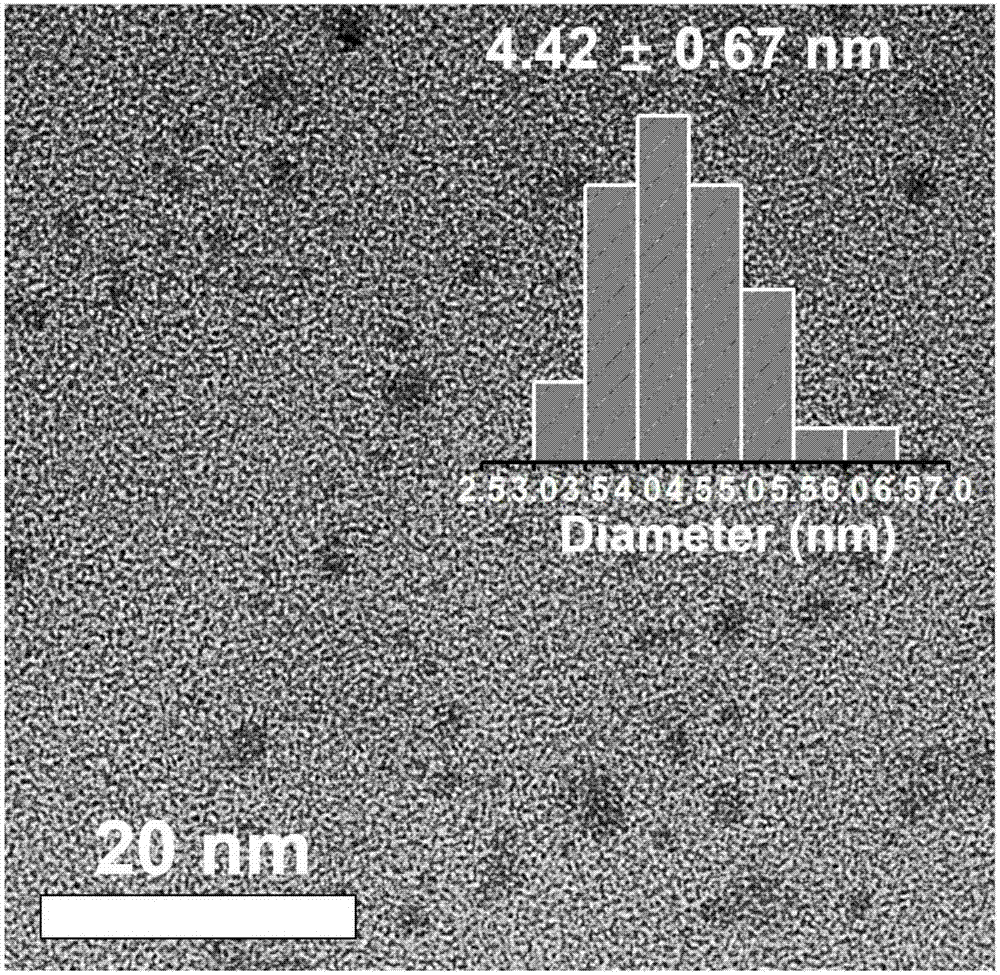
[0046] (4) Grinding the dried solid into powder with a mortar to obtain carbon dots with reddish-brown powder as red fluorescence.
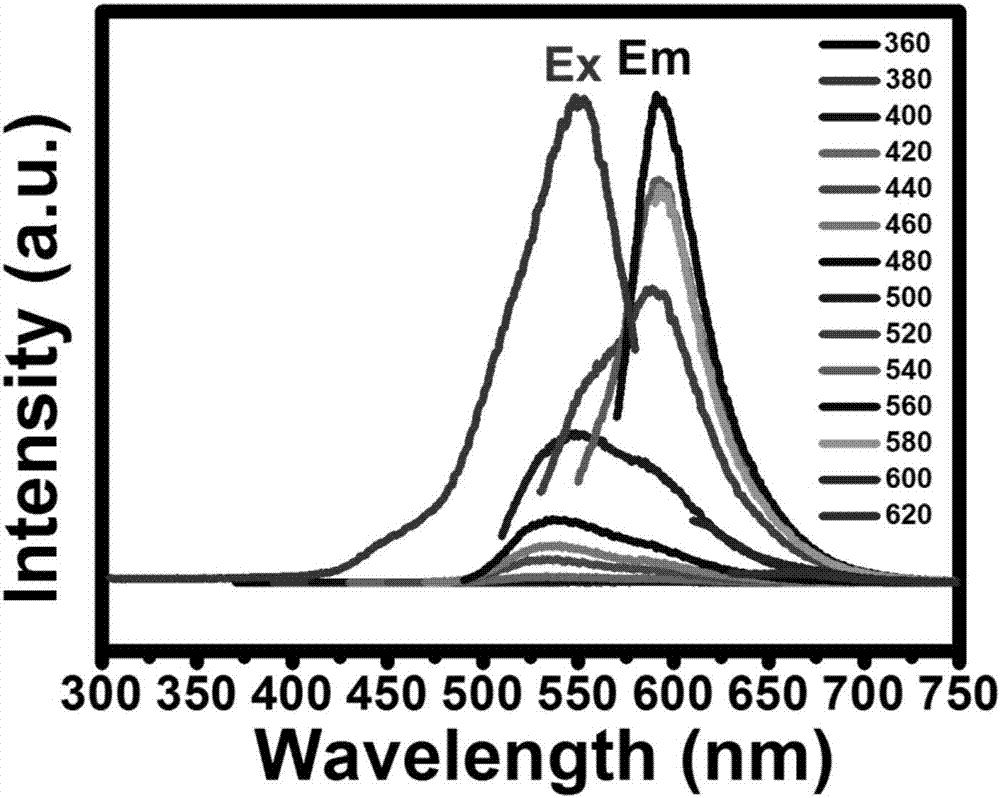
[0047] (5) A carbon quantum dot solution of 20 μg / mL was prepared, and its fluorescence properties were tested by a fluorescence spectrophotometer, and the optimum excitation wavelength was obtaine...
example 2
[0057] (1) A 20 μg / mL carbon quantum dot solution was prepared, and its fluorescence properties were tested by a fluorescence spectrophotometer, and the optimal excitation wavelength was obtained as 560 nm.
[0058] (2) Add 3 mL of the carbon quantum dot solution prepared above into the quartz fluorescence cuvette.
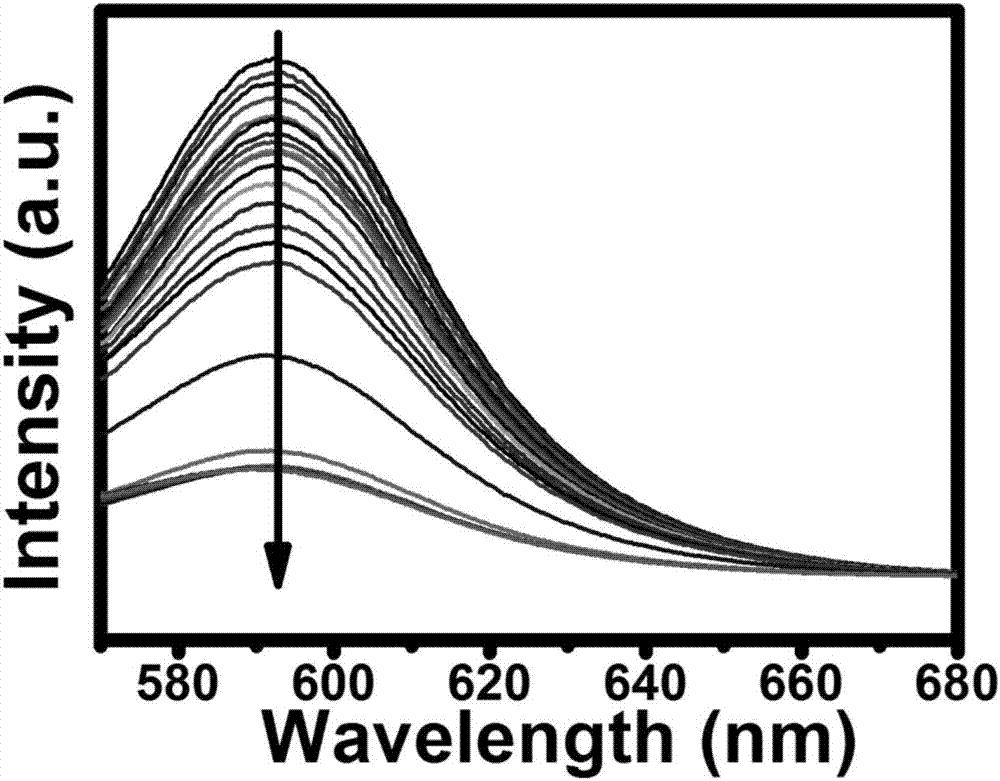
[0059] (3) Use a pipette gun to transfer FeCl 3 The solution was added to the above-mentioned carbon quantum dot solution so that the final concentration was 4 μM, stirred evenly with a pipette, and allowed to stand for 1 minute.
[0060] (4) Measure the fluorescence intensity of the above mixed solution with a fluorescence spectrophotometer, the excitation wavelength is selected as 560nm, and record the fluorescence intensity of the solution.
[0061] (5) Add ascorbic acid solutions of different concentrations to the mixed solution of carbon dots and ferric ions so that the final concentration of ascorbic acid is 0-2 μM, stir evenly with a pipette, and let stand...
example 3
[0069] (1) Add 10 μL of 1 mM Fe to 3 mL of carbon quantum dot solution containing 20 μg / mL 3+ , Li + 、Na + 、K + 、Ag + , Zn 2+ , Pb 2+ 、Ni 2+ , Mn 2+ , Fe 2+ 、Cu 2+ 、Co 2+ 、Cd 2+ 、Au 3+ 、Cr 3+ solution to obtain different mixed solutions, stirred evenly and allowed to stand for 1 minute.
[0070] (2) Measure the fluorescence intensity of the above mixed solution with a fluorescence spectrophotometer, select the excitation wavelength as 560nm, and record the change ΔF of the fluorescence intensity of the solution.
[0071] (3) from Figure 8 It can be seen that the carbon dot solution has good selectivity to ferric ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com