Cellulase mutant with high catalytic efficiency as well as coding genes and application thereof
A technology of cellulase and catalytic efficiency, applied in the field of genetic engineering, can solve the problems of low frequency of beneficial mutations, large blindness, and heavy workload of artificial mutagenesis, and achieve the goals of shortening transformation time, high catalytic efficiency, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 High Catalytic Efficiency Cellulase Mutant Encoding Gene Bacel5 127 ,Bacel5 167 clone
[0057] Using the genomic DNA of Talaromyces emersonii CBS394.64 and Bispora antennata CBS 126.38 as templates, primers were designed at the spliced region of the paternal cellulase N-terminus and the maternal cellulase C-terminus, and over-lapPCR was used to amplify the high catalytic efficiency Cellulase Mutant Encoding Gene Bacel5 127 ,Bacel5 167 .
[0058] Table 1. High catalytic efficiency cellulase mutant BaCel5 127 , BaCel5 167 Specific primers used
[0059]
[0060] a The restriction sites are underlined
Embodiment 2
[0061] Example 2 Preparation of cellulase mutants with high catalytic efficiency.
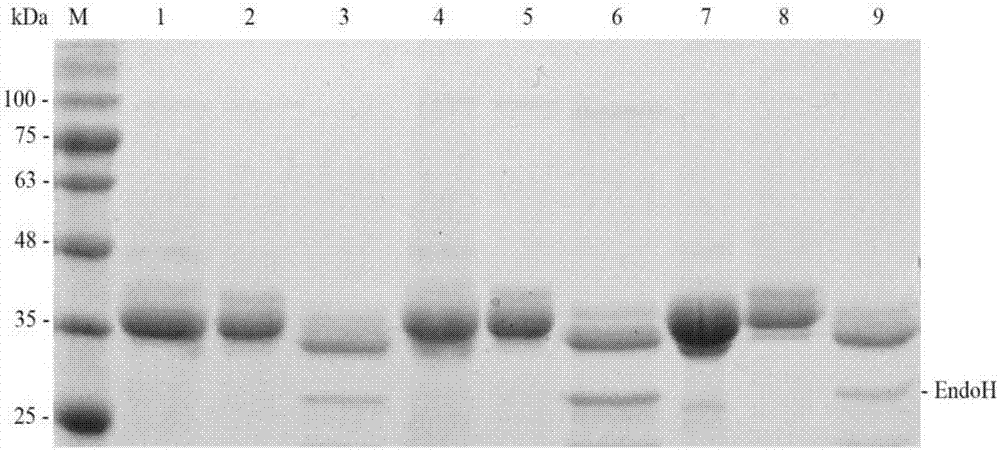
[0062] The expression vector pPIC9r was subjected to double digestion (EcoR I+Not I), and at the same time the gene Bacel5 encoding the cellulase mutant with high catalytic efficiency was 127 ,Bacel5 167 Double enzyme digestion (EcoR I+Not I), the cut gene fragment encoding the mature cellulase mutant with high catalytic efficiency (removing the signal peptide fragment) is connected with the expression vector pPIC9r, and the cellulase mutant gene containing high catalytic efficiency is obtained The recombinant plasmid pPIC9r-Bacel5 127 , pPIC9r-Bacel5 167 And transform Pichia pastoris GS115 to obtain recombinant yeast strain GS115 / Bacel5 127 ,GS115 / Bacel5 167 .
[0063] Take the GS115 strain containing the recombinant plasmid, inoculate it in a 1L Erlenmeyer flask with 300mL of BMGY medium, place it at 30°C, and culture it on a shaker at 220rpm for 48h; then centrifuge the culture solution...
Embodiment 3
[0065] Example 3 Activity Analysis of Recombinant Cellulase Mutant with High Catalytic Efficiency and Parental Wild Type
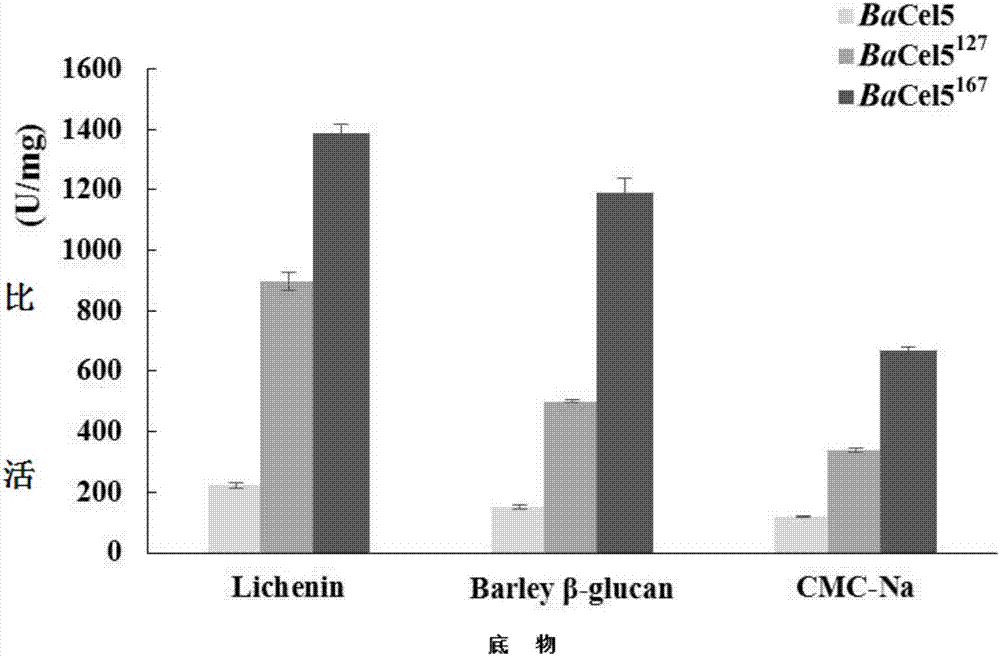
[0066] 1. DNS method: The specific method is as follows: under the given pH and temperature conditions, 1mL of the reaction system includes 100μL of appropriate diluted enzyme solution, 900μL of substrate, react for 10min, add 1.5mL of DNS to terminate the reaction, and boil for 5min. After cooling, the OD value was measured at 540 nm. Definition of cellulase activity unit: Under certain conditions, the amount of enzyme required to decompose carboxymethyl cellulose to generate 1 μmol reducing sugar per minute is 1 activity unit (U).
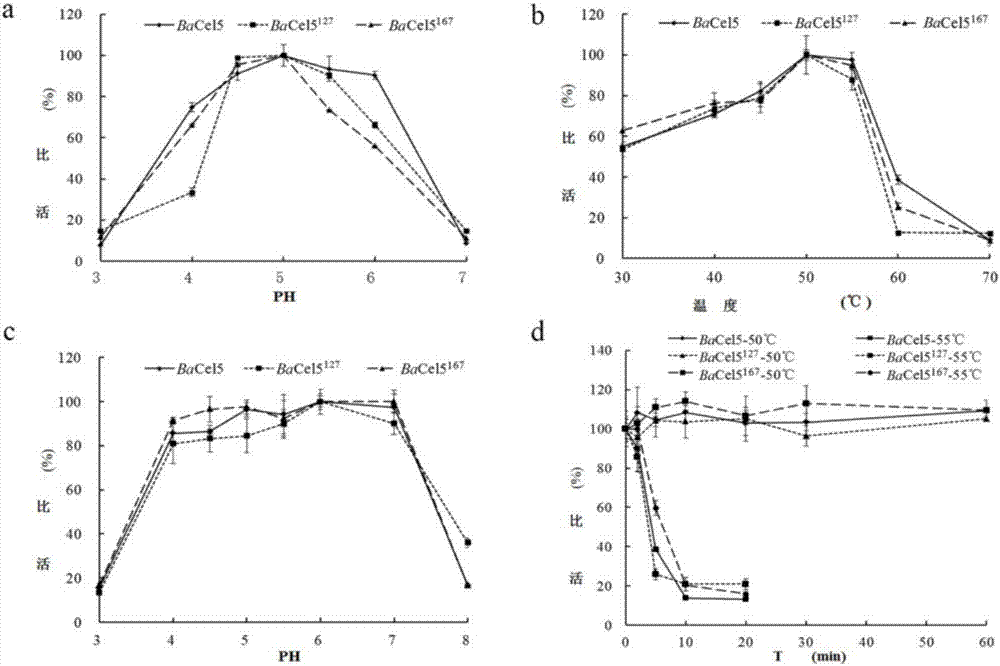
[0067] 2. Determination of the properties of the recombinant cellulase mutant with high catalytic efficiency and the parental wild type
[0068] 1. The optimum pH and pH stability determination methods of recombinant high catalytic efficiency cellulase mutant and female parent wild type are as follows:
[0069] The recombin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com