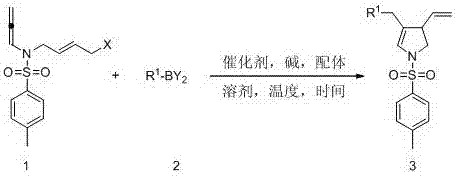
Method for compounding pyrroline derivative in one step by cyclizing and coupling suzuki
A technology for dihydropyrrole and derivatives, applied in chemical recovery, organic chemistry, etc., to achieve the effects of high yield, stable performance, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 3-vinyl-4-p-methoxybenzyl-N-p-methylbenzenesulfonyl-2,3-dihydropyrrole:
[0053] figure 2 The chemical formula of embodiment 1
[0054] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. In a nitrogen atmosphere, the substrate 1a (0.03mmol, 10mg), 2a (0.07 mmol, 10.9 mg), PdCl 2 (PPh 3 ) 2 (0.003 mmol, 2.3 mg), K 3 PO 4 (0.06mmol, 13.6 mg), weighed in turn, added to the reaction tube, evacuated to replace nitrogen, and added ethylene glycol dimethyl ether and dioxane (1V:15V) (total 2 mL) under nitrogen atmosphere. The reaction system was heated to 50°C and reacted for 10 hours. After the reaction was detected by TLC, the system was cooled to room temperature. Silica gel was added directly, spin-dried and column chromatographed to obtain light yellow paste 3aa (97%). 1 H NMR (400 MHz, CDCl 3 , δ ppm): 7.66–7.61 (m, 2H), 7.35–7.31 (m, 2H), 6.97–6.94 (m,2H), 6.82–6.78 (m, 2H), 6.01 (q, J = 1.6 Hz, 1H), 5.26 (ddd, J = 16.9 Hz,10.0 Hz, 8....
Embodiment 2
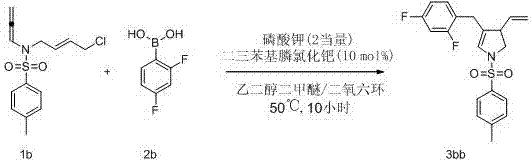
[0056] image 3 The chemical formula of embodiment 2
[0057] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. In a nitrogen atmosphere, the substrate 1b (0.03mmol, 8.9mg), 2b (0.07 mmol, 10.9 mg), PdCl 2 (PPh 3 ) 2 (0.003 mmol, 2.3 mg), K 3 PO 4 (0.06mmol, 13.6 mg), weighed in turn, added to the reaction tube, evacuated to replace nitrogen, and added ethylene glycol dimethyl ether and dioxane (1V:15V) (total 2 mL) under nitrogen atmosphere. The reaction system was heated to 50°C and reacted for 10 hours. After the reaction was detected by TLC, the system was cooled to room temperature. Silica gel was added directly, spin-dried and column chromatographed to obtain light yellow paste 3bb (98%). 1 H NMR (400 MHz, CDCl 3 , δ ppm): 7.63–7.57 (m, 2H), 7.32 (d, J = 8.0 Hz, 2H), 7.00(td, J = 8.5 Hz, 6.5 Hz, 1H), 6.82–6.73 (m, 2H), 6.00 (q, J = 1.6 Hz, 1H),5.25 (ddd, J = 16.8 Hz, 10.1 Hz, 8.7 Hz, 1H), 4.98–4.88 (m, 2H), 3.67 (dd, J = 10.6 Hz, 9...
Embodiment 3
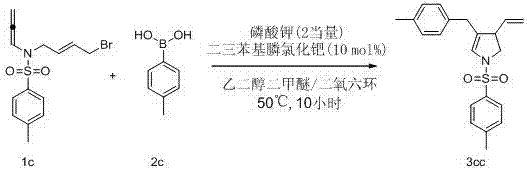
[0059] Figure 4 The chemical equation of embodiment 3
[0060] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. In a nitrogen atmosphere, the substrate 1c (0.2mmol, 74.5mg), 2c (0.5 mmol, 68.1 mg), PdCl 2 (PPh 3 ) 2 (0.02 mmol, 14 mg), K 3 PO 4 (0.4mmol, 84.9 mg), weighed in turn, added to the reaction tube, evacuated to replace nitrogen, and added ethylene glycol dimethyl ether and dioxane (1V:15V) (total 4 mL) under nitrogen atmosphere. The reaction system was heated to 50°C and reacted for 10 hours. After the reaction was detected by TLC, the system was cooled to room temperature. Directly add silica gel, spin dry column chromatography, obtain light yellow paste 3cc (93%). 1 H NMR (400 MHz, CDCl 3 , δ ppm): 7.66–7.61 (m, 2H), 7.33 (d, J = 8.0 Hz, 2H), 7.07(d, J = 7.8 Hz, 2H), 6.94 (d, J = 7.9 Hz, 2H), 6.03 (q, J = 1.6 Hz, 1H), 5.27(ddd, J = 16.9 Hz, 10.1 Hz, 8.9 Hz, 1H), 4.98–4.85 (m, 2H), 3.66 (dd, J =10.9 Hz, 9.8 Hz, 1H), 3.29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com