Fusion protein containing KLH (Keyhole Limpet Haemocyanin) fragment and application thereof
A keyhole limpet hemocyanin and fusion protein technology, which is applied to medical preparations containing active ingredients, fusion polypeptides, DNA/RNA fragments, etc., can solve the problems of keyhole limpet hemocyanin length and increase immunogenicity, and achieve Strong immunogenicity, enhanced immunogenicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 sKLH fragment gene synthesis and NAD4 gene cloning
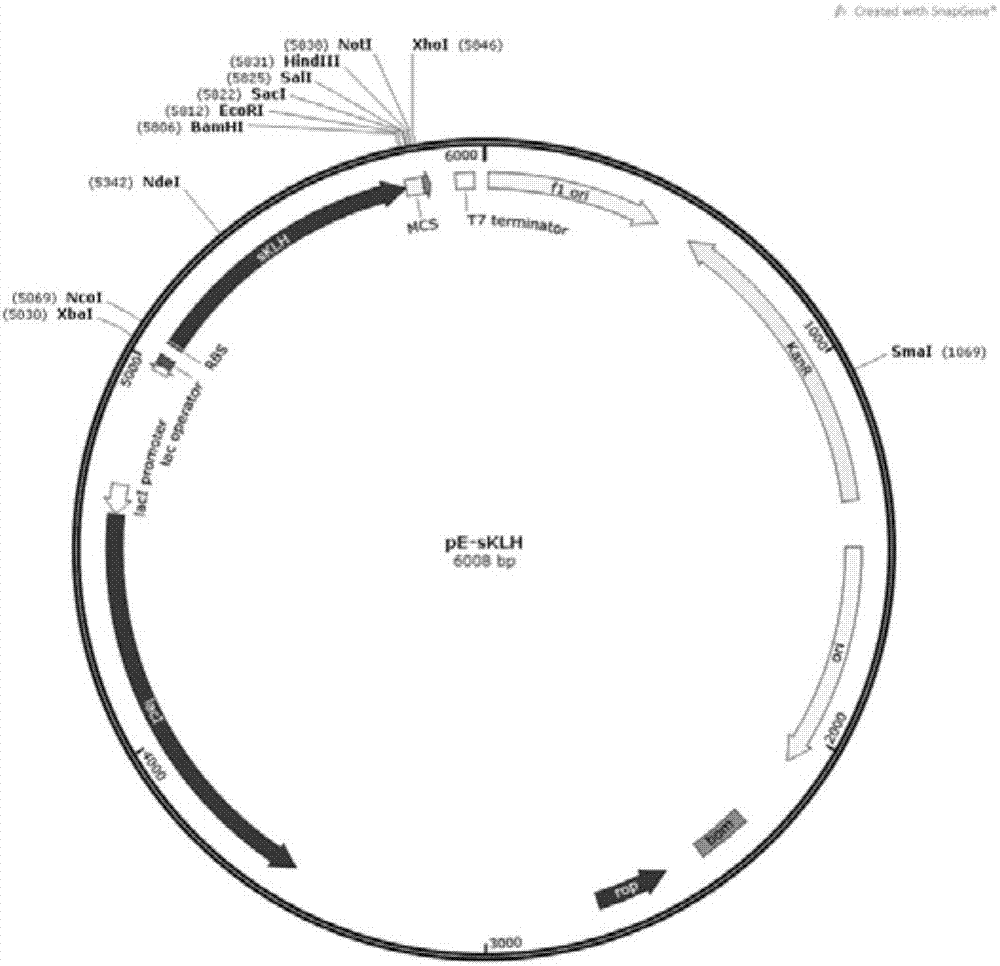
[0030] The sKLH fragment gene was synthesized according to the keyhole limpet hemocyanin protein gene coding sequence in the GenBank database (Accession Number: AJ698341.2, its nucleotide sequence is shown in SEQ ID NO: 2), and at the same time according to the E.coli genetic code preference The gene sequence of the sKLH fragment (amino acids 1-245) was codon-optimized, and pUC57-sKLH plasmid was synthesized and constructed by Nanjing GenScript Co., Ltd., and the nucleic acid sequence after codon optimization and adding restriction sites is shown in SEQ ID NO:3 shown.
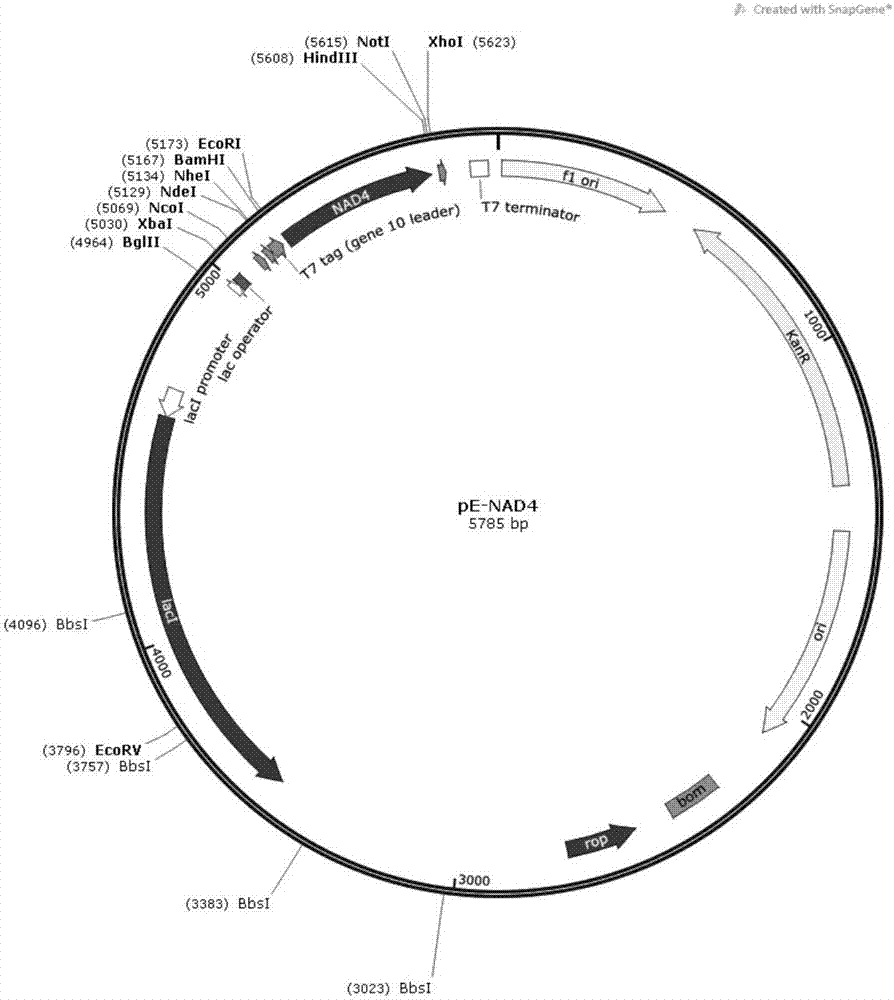
[0031] NAD4 gene clone Arabidopsis leaf tissue was ground with liquid nitrogen and extracted with Trizol (Invitrogen) to obtain RNA, and then III Reverse transcriptase treatment to obtain cDNA. Using cDNA as a template, the nucleotide sequence of NAD4 gene (as shown in SEQ ID NO: 4) was obtained by high-fidelity PCR. The design of the amp...
Embodiment 2
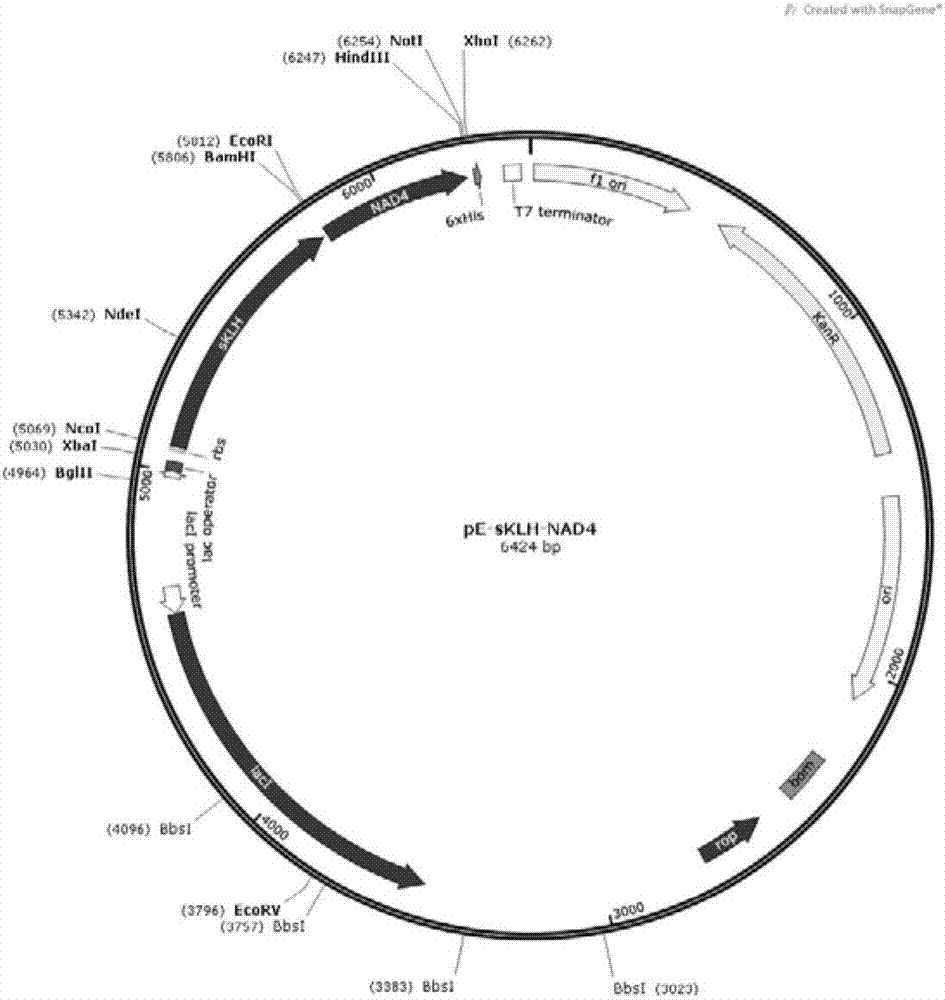
[0034] Example 2 Construction of protein expression vector pE-sKLH-NAD4 plasmid
[0035] Construction of the pE-sKLH plasmid Plasmid pUC57-sKLH (prepared in Example 1) was digested with NcoI and BamHI double enzymes (NEB), the gel recovery enzyme digested the gene containing the sKLH fragment (~740bp), and similarly digested with NcoI and BamHI double enzymes The pET-28a plasmid recovered from the gel was ligated, transformed into DH5α competent bacteria, positive clones were screened on a karimycin resistance plate, and the pE-sKLH plasmid was identified by sequencing with T7 primers.
[0036] Construction of the pE-NAD4 plasmid The NAD4 gene nucleotide sequence obtained by high-fidelity PCR (prepared in Example 1) was digested with EcoRI and HindIII (NEB), and the NAD4 nucleic acid fragment (~430bp) was digested by gel recovery, and the same The pET-28a plasmids recovered by EcoRI and HindIII double-enzyme gels were ligated, transformed into DH5α competent bacteria, positive...
Embodiment 3
[0038] Example 3 Expression and purification of sKLH-NAD4 fusion protein
[0039] Pick single colonies containing pE-NAD4 and pE-sKLH-NAD4 plasmids from the LB plate containing kanamycin, inoculate them in 10ml liquid LB medium containing kanamycin, and culture overnight at 37°C with shaking at 180rpm , Take 5ml and transfer it to 500mL LB culture medium containing kanamycin, shake and cultivate at 37°C at 180rpm for 4-5 hours. When the OD600 reached about 0.6, IPTG was added to a final concentration of 0.5mM, and induced by shaking at 37°C for 4 hours. After induction, the cells were collected by centrifugation at 8000 g for 10 min, then suspended in 20 ml of lysate (20 mM Tris-HCl, pH 7.6, 300 mM NaCl, 5 mM EDTA) corresponding to 1 g of cells, and homogeneously crushed twice under high pressure at 600 bar. Centrifuge at 12000g for 30min, discard the supernatant and keep the precipitate (ie inclusion body). Then resuspend the pellet with resuspension solution (20mM Tris-HCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com