2,3,4,5-tetrahydropyridin-6-amine and 3,4-dihydro-2h-pyrrol-5-amine compound inhibitors of beta-secretase
An organic light-emitting device and compound technology, applied in the field of organic light-emitting devices, can solve the problems of difficulty in practical application, high driving voltage, low efficiency, etc., and achieve the effect of achieving high efficiency and long life, ensuring low voltage driving and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0248] Fabrication of Organic Light Emitting Devices
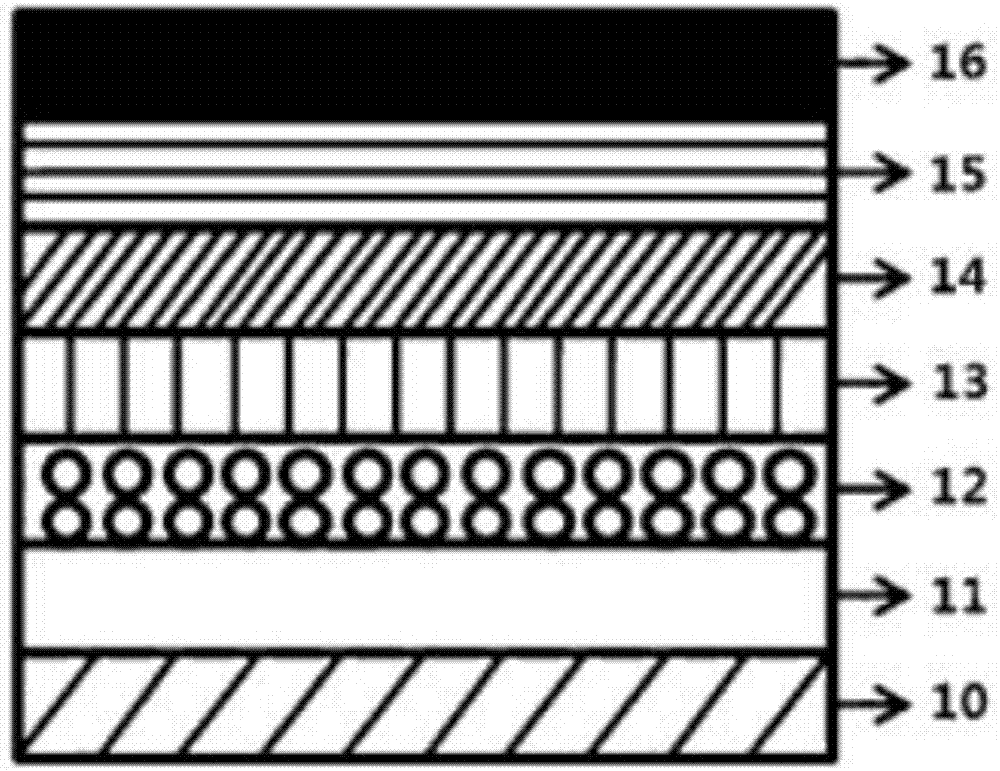
[0249] according to figure 1 The structure shown produces an organic light-emitting device. The organic light-emitting device is stacked sequentially from bottom to top with anode (hole injection electrode 11) / hole injection layer 12 / hole transport layer 13 / light emitting layer 14 / electron transport layer 15 / cathode (electron injection electrode 16).
[0250] The following substances were used for the hole injection layer 12, the hole transport layer 13, the light emitting layer 14, and the electron transport layer 15 of the examples and comparative examples.
[0251]
[0252] Before fabricating an organic light-emitting device, in order to observe that the energy formed by the exciplex is effectively transferred to the combination of host 1 and host 2 of the phosphorescent dopant, host 1 / host 2 (1:1) was evaporated on a glass substrate, and measured The exciplex wavelengths are shown in Table 1 below.
[0253] Table...
Embodiment 1
[0259] Ultrasonic cleaning with distilled water A glass substrate coated with a thin film of indium tin oxide (ITO). Once the distilled water cleaning is completed, ultrasonic cleaning and drying are performed with solvents such as isopropanol, acetone, and methanol, and then transferred to a plasma cleaner. After cleaning the above-mentioned substrate with oxygen plasma for 5 minutes, use a thermal evaporation coater (thermalevaporator). With hole injection layer HI01 Hole transport layer, on top of ITO substrate, with NPB Make a film. Afterwards, as the above-mentioned light-emitting layer, doping compound 1-1 / Ir(ppy) 3 10% to Make a film. Then, as the electron transport layer, with ET01:Liq(1:1) After film formation, LiF Aluminum (Al) A film was formed, and the device was sealed (encapsulated) in a glove box, thereby producing a green organic light-emitting device.
Embodiment 2
[0261] A green organic light-emitting device was fabricated by the same method except that Compound 1-2 was used as the host of the light-emitting layer in Example 1 above instead of Compound 1-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com