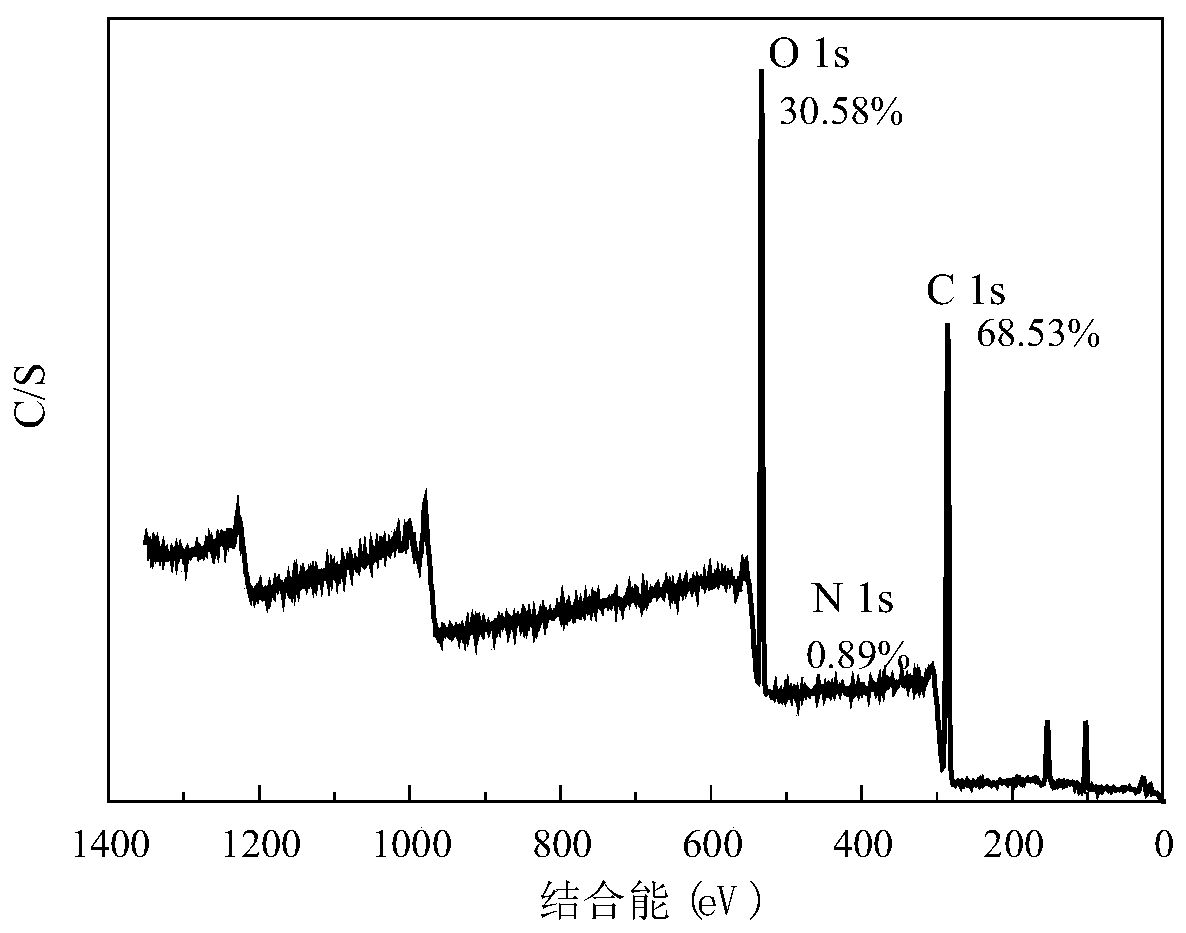
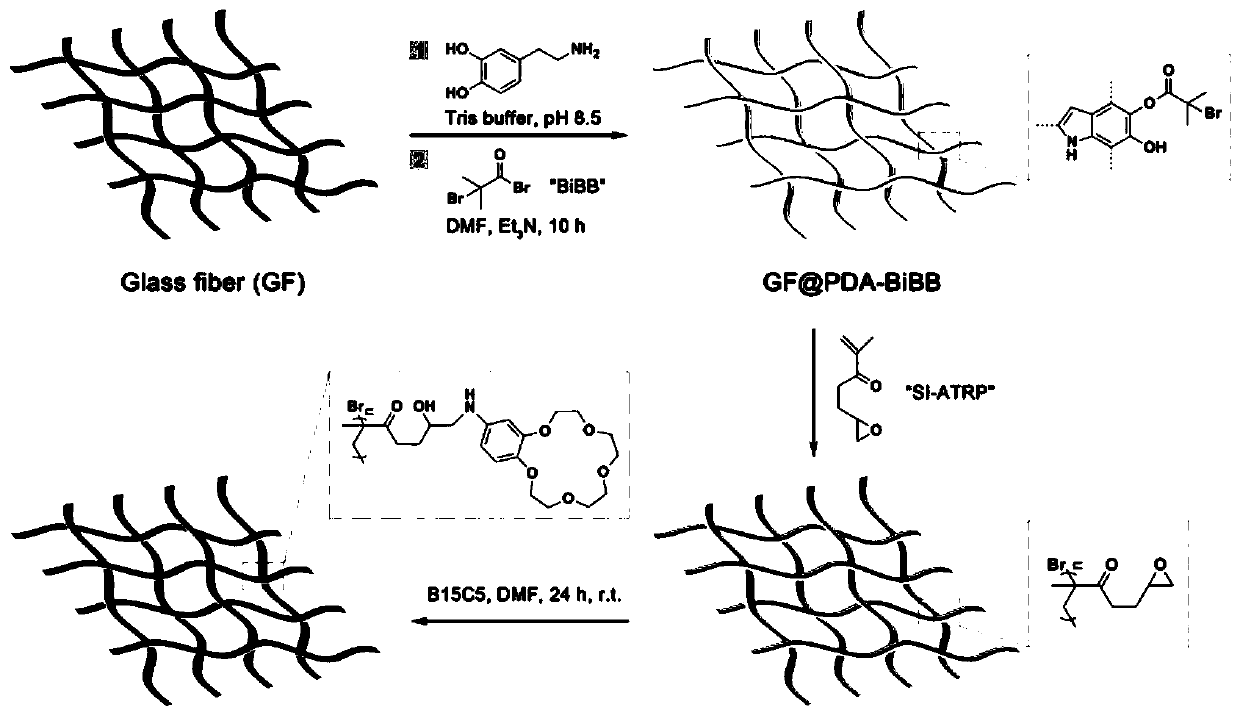
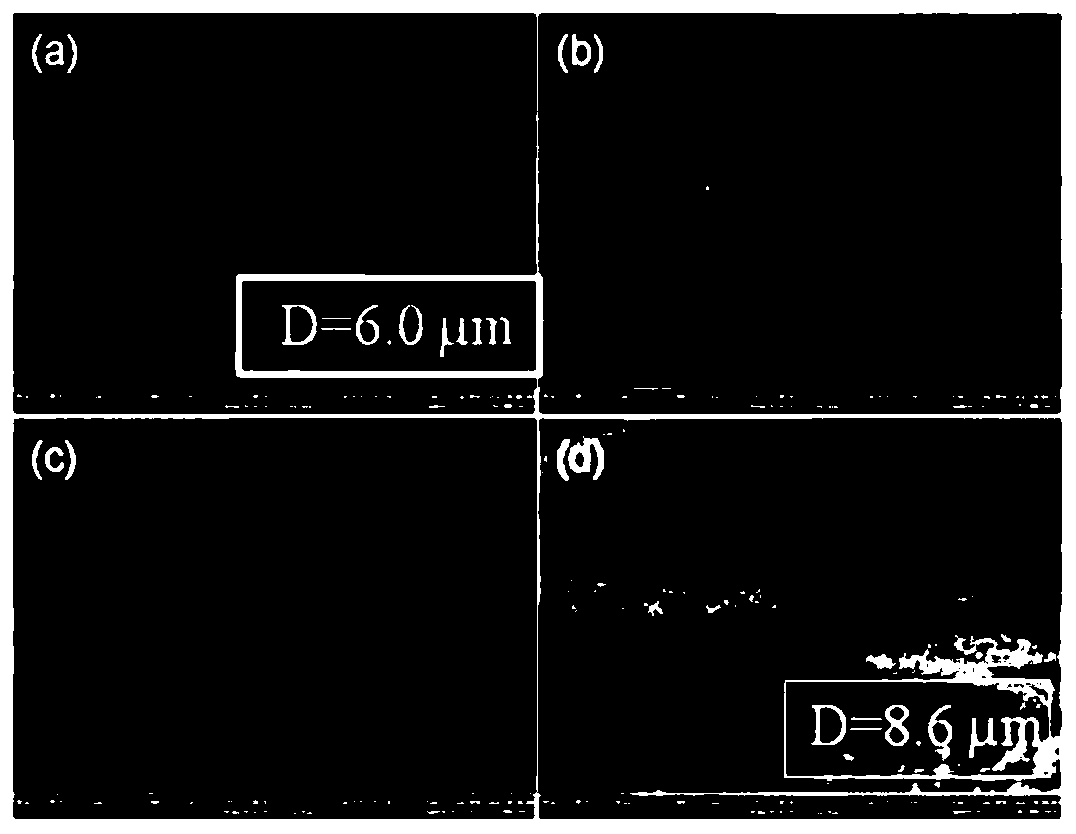
A kind of crown ether immobilized glass fiber material and preparation method thereof
A glass fiber and crown ether technology is applied in the field of crown ether immobilized glass fiber material and its preparation. Simple and convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Cut 2×4cm 2 Add the original glass fiber mat to the Tris salt solution of 2g / L dopamine, shake at constant temperature for 24h, wash and dry repeatedly with deionized water and ethanol; put the above glass fiber mat material in 60mL DMF, add 1.5mL triethyl ether Add 1.5mL of 2-bromoisobutyryl bromide under the protection of inert gas, react at room temperature for 12h, wash and dry repeatedly with DMF and ethanol; put the above glass fiber mat material in 3.0mL of anisole, add 3.0mL of methyl Glycidyl acrylate, 33.0 μL ethyl 2-bromoisobutyrate and 740 μL azobisisobutyronitrile in DMF (concentration 1.0 mg / L), under the protection of an inert gas atmosphere, add 1.0 mL copper bromide and tris DMF solution of (2-dipicolylmethyl)amine (concentrations of the two are 1.0 and 3.0mg / L respectively), 60 ℃ oil bath for 24h, THF, DMF and ethanol repeatedly washed and dried; the above-mentioned glass fiber mat material was placed in 4-Aminobenzo15-crown-5 in DMF solution (concent...
Embodiment 2
[0038] Cut 2×4cm 2 Add the original glass fiber mat to the Tris salt solution of 2g / L dopamine, shake at constant temperature for 24h, wash and dry repeatedly with deionized water and ethanol; put the above glass fiber mat material in 60mL DMF, add 1.5mL triethyl ether Add 1.5mL of 2-bromoisobutyryl bromide under the protection of inert gas, react at room temperature for 12h, wash and dry repeatedly with DMF and ethanol; put the above glass fiber mat material in 3.0mL of anisole, add 3.0mL of methyl Glycidyl acrylate, 33.0 μL ethyl 2-bromoisobutyrate and 1.0 mL ascorbic acid in DMF (concentration 3.97 mg / L), under the protection of an inert gas atmosphere, add 1.0 mL copper bromide and pentamethyldiethylene The DMF solution of triamine (both concentrations are 1.0 and 2.3mg / L respectively), 60 ℃ oil bath 24h, THF, DMF and ethanol wash repeatedly and dry; Crown-5 in DMF solution (concentration: 25g / L), reflux reaction at 70°C for 6h, repeated washing with DMF and ethanol, and ...
Embodiment 3
[0040] Cut 2×4cm 2 Add the original glass fiber mat to the Tris salt solution of 2g / L dopamine, shake at constant temperature for 24h, wash and dry repeatedly with deionized water and ethanol; put the above glass fiber mat material in 60mL DMF, add 1.5mL triethyl ether Add 1.5mL of 2-bromoisobutyryl bromide under the protection of inert gas, react at room temperature for 12h, wash and dry repeatedly with DMF and ethanol; put the above glass fiber mat material in 3.0mL of anisole, add 3.0mL of methyl Glycidyl acrylate, 33.0 μL ethyl 2-bromoisobutyrate and 740 μL azobisisobutyronitrile in DMF (concentration 1.0 mg / L), under the protection of an inert gas atmosphere, add 1.0 mL copper bromide and tris DMF solution of (2-dipicolylmethyl)amine (concentrations of the two are 1.0 and 3.0mg / L respectively), 60 ℃ oil bath for 24h, THF, DMF and ethanol repeatedly washed and dried; the above-mentioned glass fiber mat material was placed in 4-Aminobenzo-18-crown-6 in DMF solution (concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com