Fatty acid interference-resistant anti-human heart-type fatty acid binding protein monoclonal antibody and application thereof
A monoclonal antibody and fatty acid combination technology, applied in the direction of anti-animal/human immunoglobulin, antibody, immunoglobulin, etc., to achieve good temperature stability, good affinity and specificity, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Expression purification and purity identification of embodiment 1 recombinant H-FABP protein
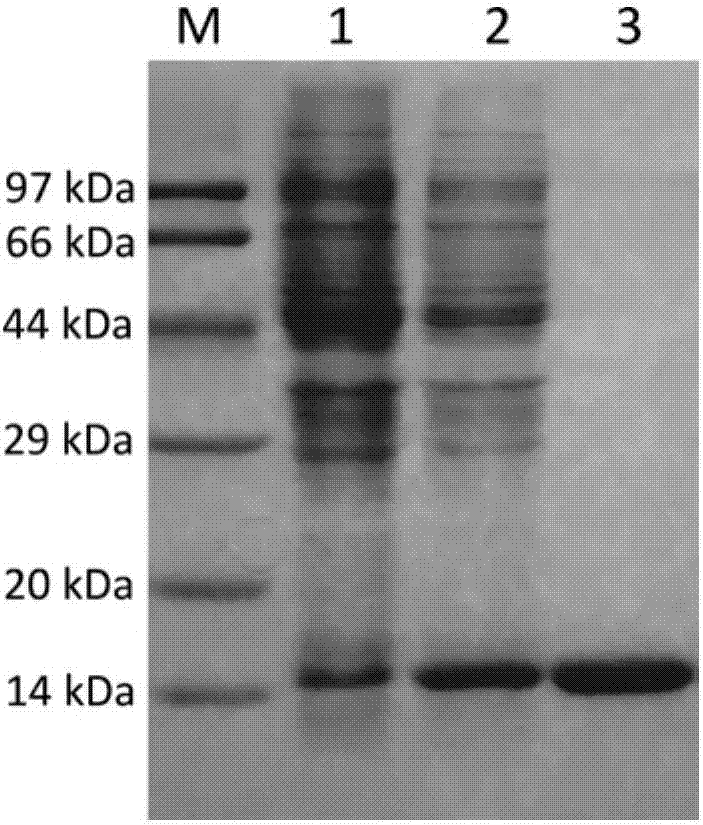
[0049] The prokaryotic expression plasmid containing the H-FABP gene sequence was transformed into Escherichia coli BL21(DE3), and cultured on LB plates. Pick a single colony from the plate and inoculate it into LB medium. After expanding the culture, when the OD600 value of the bacterial solution reaches 0.6-0.8, add IPTG with a final concentration of 1 mM to induce expression. After 4 hours, the bacterial cells were collected by centrifugation, and the supernatant was collected by centrifugation after the bacteria were disrupted by ultrasonication. The supernatant was purified by His-taq column and molecular sieve chromatography column, and the samples were collected and identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for protein purity.
[0050] After electrophoresis, a target protein band with a molecular weight of about 15 kDa was observed on the ...
Embodiment 2
[0051] Example 2 Animal immunity and serum titer detection
[0052] The purified recombinant H-FABP protein with a concentration of 1mg / ml was emulsified with the same amount of Freund's complete adjuvant and Freund's incomplete adjuvant, and the emulsification method used the double syringe push method. 6-8 week specific pathogen-free grade female BALB / c mice were injected with immune antigen H-FABP emulsified in Freund's complete adjuvant to each mouse from the hock joint with 40 μg of antigen, 2 days later, with Freund's incomplete adjuvant The emulsified immune antigen H-FABP was injected into each mouse with 40 μg antigen again from the hock joint. Eight days later, blood was collected from the tail vein, centrifuged to obtain the supernatant, and the serum titer was detected by indirect ELISA with the pre-immune serum as a negative control. After the second immunization, the serum titer was as high as 2.0 or more after a million-fold dilution (Table 1). Select the serum...
Embodiment 3
[0055] Example 3 Cell Fusion, Positive Hybridoma Screening and Subcloning
[0056] 1. Preparation and screening of high-titer hybridoma cell lines
[0057] Sp2 / 0 myeloma cells in good growth state were selected and mixed with lymphocytes of immunized mice at a ratio of 1:2-1:3, and PEG1500 preheated at 37°C was used as a fusion agent. The fused cells were cultured in HAT-1640 medium containing 20% FBS serum. After 5-7 days, observe the fusion effect and change the medium, and take the cell culture medium 3 days after the medium change. Use the serum of the patient within 6-12 hours of clinically confirmed myocardial infarction to coat the ELISA plate. The serum contains a high concentration of natural H-FABP protein. Use the indirect ELISA method to screen positive clones, and select the one with a higher ratio of positive value to cell number. The cells were subcloned multiple times, and finally a hybridoma cell library capable of stably secreting monoclonal antibodies ag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com