A kind of pyrene-based organic semiconductor laser material and its preparation method and application
A technology of organic semiconductors and laser materials, applied in the field of pyrene-based organic semiconductor laser materials and their preparation, can solve the problems of effective control of electrical properties, high threshold value of optical gain medium, complex material preparation, etc., and achieve excellent thermal stability, preparation method Simple, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
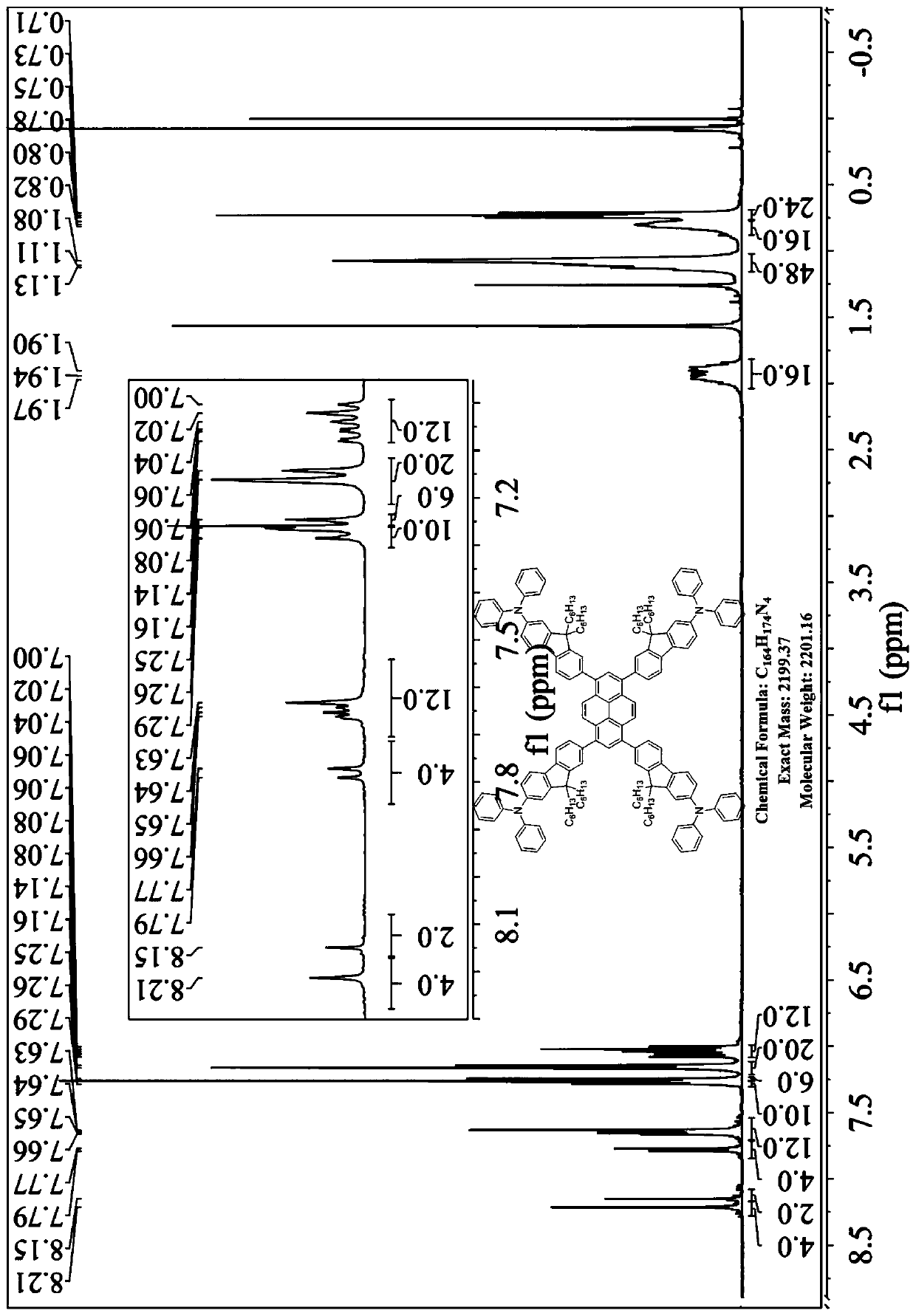
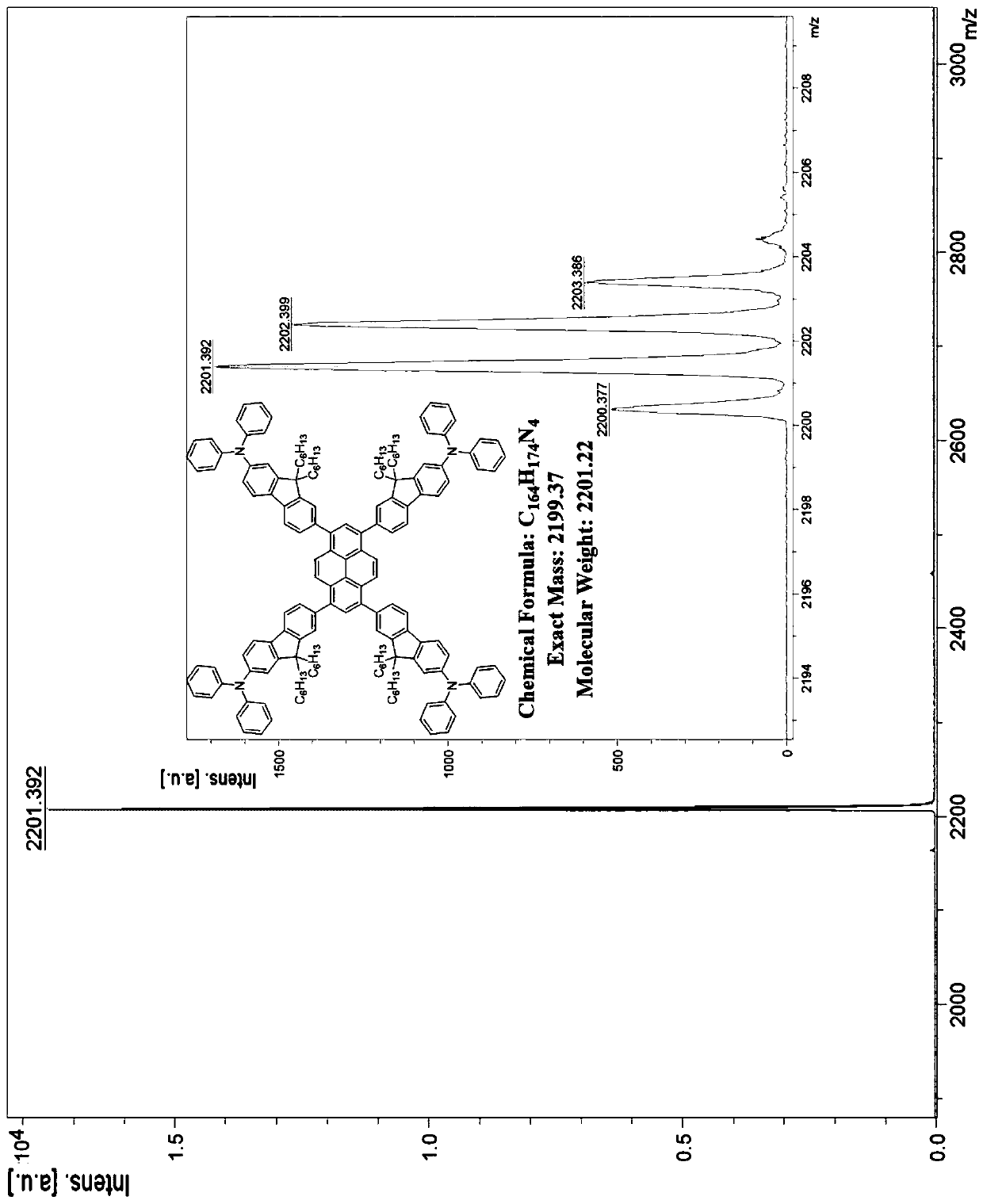
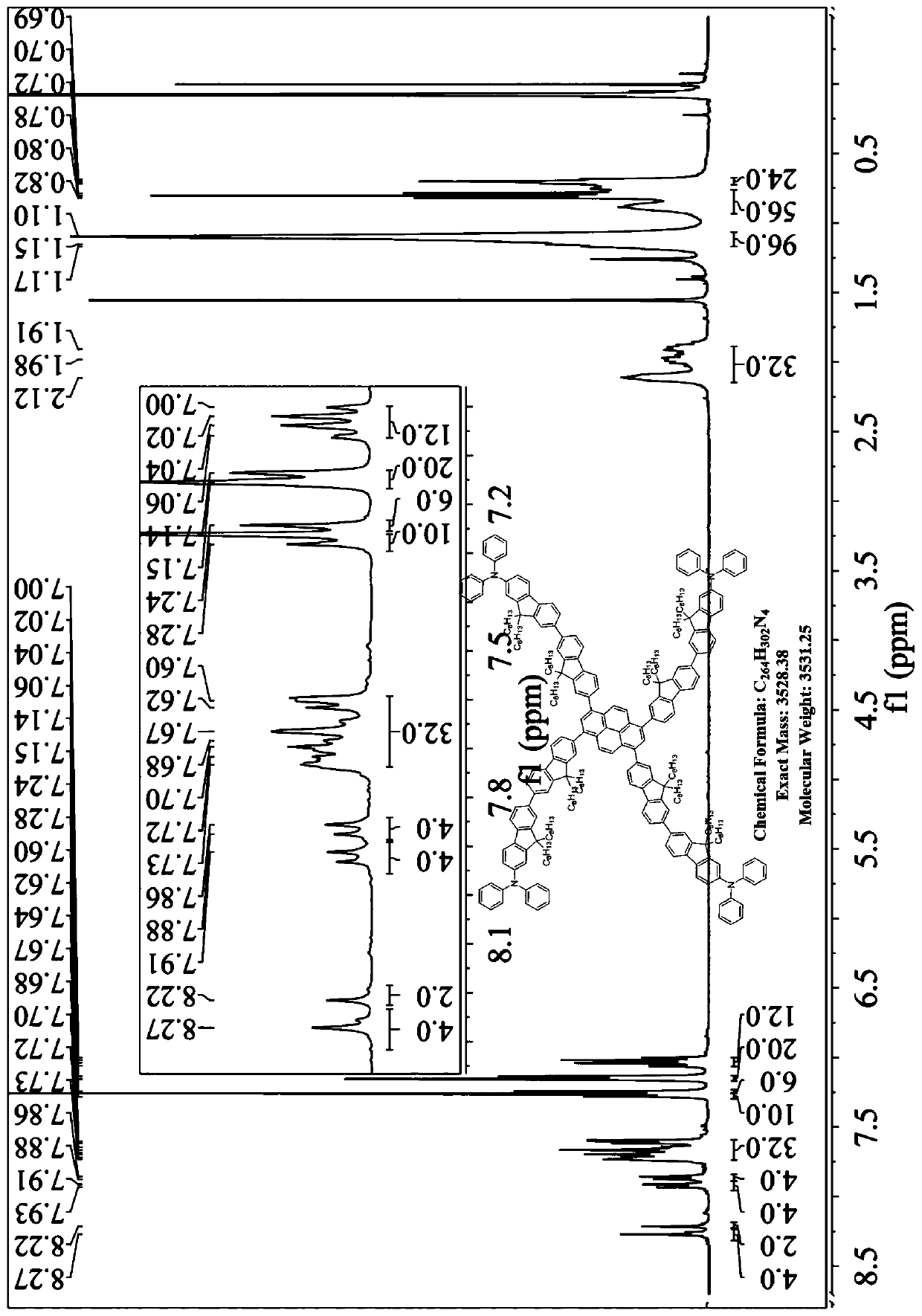
[0024] The preparation method of the present invention comprises: chemically reacting diphenylamine with 2,7-dibromofluorene to obtain a monobromo-substituted derivative of fluorene modified with a unilateral diphenylamine end group, then carrying out a boron esterification reaction, and finally combining it with pyrene Tetrabromonucleus was prepared by Suzuki coupling reaction to obtain compound P n F. This kind of four-substituted butterfly-shaped organic semiconductor laser material based on diphenylamine terminal group modification with pyrene as the core and fluorene chain as the arm has low threshold, high optical gain coefficient, high thermal stability and excellent electroluminescence performance. It has the following general structural formula:
[0025]
[0026] Wherein, R is a C1-C30 linear or branched alkyl or alkoxy group; n is a natural number of 1-5; N is a nitrogen atom; H is a hydrogen atom.
[0027] More specifically, said R may be one of methyl, ethy...
Embodiment 1
[0033]
[0034] Reaction condition one: in N 2 Under protective conditions, diphenylamine (169.1mg, 1mmol), 9,9-dihexyl-2,7-dibromofluorene (980.2mg, 2.0mmol), potassium tert-butoxide (179.5mg, 1.6mmol), iodide Cuprous catalyst (9.5mg, 0.05mmol) and phase transfer catalyst (0.5mL) was dissolved in 80mL of anhydrous 1,4-dioxane solution, under the condition of temperature control at 110°C, protected from light with tinfoil, reacted for 48h; after the reaction was completed, cooled to room temperature, extracted and purified by chromatographic column to obtain the reaction Product 2a (324.9 mg), yield 56.1%.
[0035] Reaction condition two: in N 2 Under protective conditions, diphenylamine (169.1mg, 1mmol), 9,9-dihexyl-2,7-dibromofluorene (1.2g, 2.5mmol), potassium tert-butoxide (202.1mg, 1.8mmol), iodide Cuprous catalyst (11.4mg, 0.06mmol) and phase transfer catalyst (0.4mL) was dissolved in 80mL of anhydrous 1,4-dioxane solution, under the condition of temperature con...
Embodiment 2
[0039]
[0040] Reaction condition one: in N 2 Under protective conditions, the reaction product 2a (5.8g, 10mmol) was dissolved in 80mL of tetrahydrofuran solvent that had been dried by bubbling, and the reaction device was placed in an ice-water bath (acetone+dry ice) at -78°C, and 7.8mL of n-C 4 h 9 Li is added to the reaction device in small amounts several times; the n-C 4 h 9 After all the Li was injected (1.5h), 4.5mL of isopropanol pinacol borate was added; under temperature control at 0°C, the reaction was carried out for 12h; after the reaction was completed, it was cooled to room temperature and purified by extraction and chromatographic column to obtain the reaction product 3a ( 2.5 g), yield 40.1%.
[0041] Reaction condition two: in N 2 Under protective conditions, the reaction product 2a (8.7g, 15mmol) was dissolved in 85mL of tetrahydrofuran solvent that had been bubbled and dried, and the reaction device was placed in an ice-water bath (acetone+dry ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com