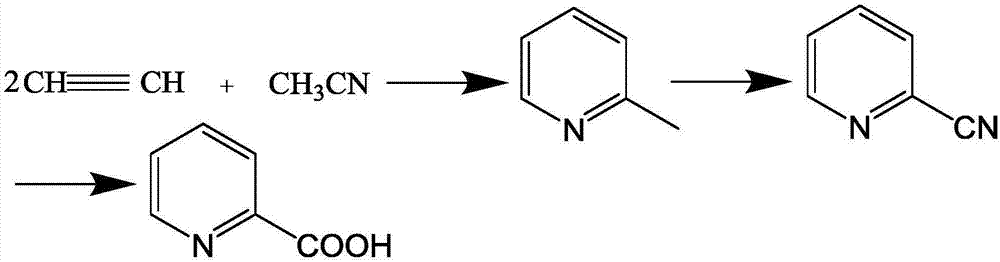
Synthetic method for 2-picolinic acid
A technology of picolinic acid and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of a large amount of waste liquid, pollute the environment, and equipment corrosion, and achieve the effects of less by-products, lower reaction temperature, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1, a kind of synthetic method of 2-picolinic acid, comprises the following steps:
[0027] (1) Dicyclopentadienyl cobalt, manganese acetate and cobalt acetate are added to the reactor with a mass ratio of 10:2:1 as a catalyst and acetonitrile, and dry acetylene is introduced at room temperature until the pressure of the reactor rises to 1.1MPa, Stir and heat up to 145°C, react for 9.5 hours, concentrate the reaction solution, and purify by rectification to obtain 2-picoline;
[0028] (2) Heating the above-mentioned 2-picoline on a heating bed to 350-370°C to turn into steam, then mix it with ammonia, water vapor and air, and catalyze oxidation through the catalyst layer to obtain 2-pyridinecarbonitrile, The catalyst layer includes a catalyst, a promoter and a catalyst carrier, the catalyst is germanium oxide, antimony trioxide and vanadium oxide, the promoter is copper and zirconium, and the catalyst carrier is alumina;
[0029] (3) Add the above-mentioned 2-pyridineca...
Embodiment 2
[0031] 1, a kind of synthetic method of 2-picolinic acid, comprises the following steps:
[0032] (1) Add dicyclopentadienyl cobalt, manganese acetate and cobalt acetate into the reactor with a mass ratio of 10:2:1 as a catalyst and acetonitrile, feed dry acetylene at room temperature until the pressure of the reactor rises to 1MPa, stir Raise the temperature to 150°C, react for 9 hours, concentrate the reaction solution, and purify by rectification to obtain 2-picoline;
[0033] (2) Heating the above-mentioned 2-picoline on a heating bed to 350-370°C to turn into steam, then mix it with ammonia, water vapor and air, and catalyze oxidation through the catalyst layer to obtain 2-pyridinecarbonitrile, The catalyst layer includes a catalyst, a promoter and a catalyst carrier, the catalyst is germanium oxide, antimony trioxide and vanadium oxide, the promoter is copper and zirconium, and the catalyst carrier is alumina;
[0034] (3) Add the above-mentioned 2-pyridinecarbonitrile ...
Embodiment 3
[0036] Substantially the same as Example 1, the difference is that the reactor pressure is 0.8MPa in step (1), the temperature is 140°C, and the reaction is 8h; the concentration of sodium hydroxide solution in step (3) is 40%, and the temperature is raised to 70°C The reaction was stirred for 5h, cooled to room temperature and then stirred for 5h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com