In-vitro amplification method of cord blood Treg cells
An in vitro expansion and cell technology, applied in the field of in vitro expansion of cord blood Treg cells, can solve the problems of unsatisfactory expansion multiples and expansion cycles, cumbersome operations, increased cell pollution, etc., to achieve immunosuppressive functions, simplifying Amplification method, the effect of a wide range of cord blood sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The process of separating cord blood mononuclear cells is as follows: neonatal cord blood is collected from full-term healthy newborns in the Department of Obstetrics and Gynecology. After the fetus is delivered, 100ml of blood is collected from the placenta umbilical vein and placed in a blood collection bag containing citric acid anticoagulant. The blood was sent to the laboratory for processing within 4 hours after collection. Take 100ml of cord blood and centrifuge at 750g / min for 15min. Collect the upper layer of plasma, inactivate the plasma at 56°C for 30 min, centrifuge at 2500 rpm / min and use it for later use; add an equal volume (1:1) of PBS to dilute the remaining blood cells, and separate mononuclear cells by conventional density gradient centrifugation.
Embodiment 2
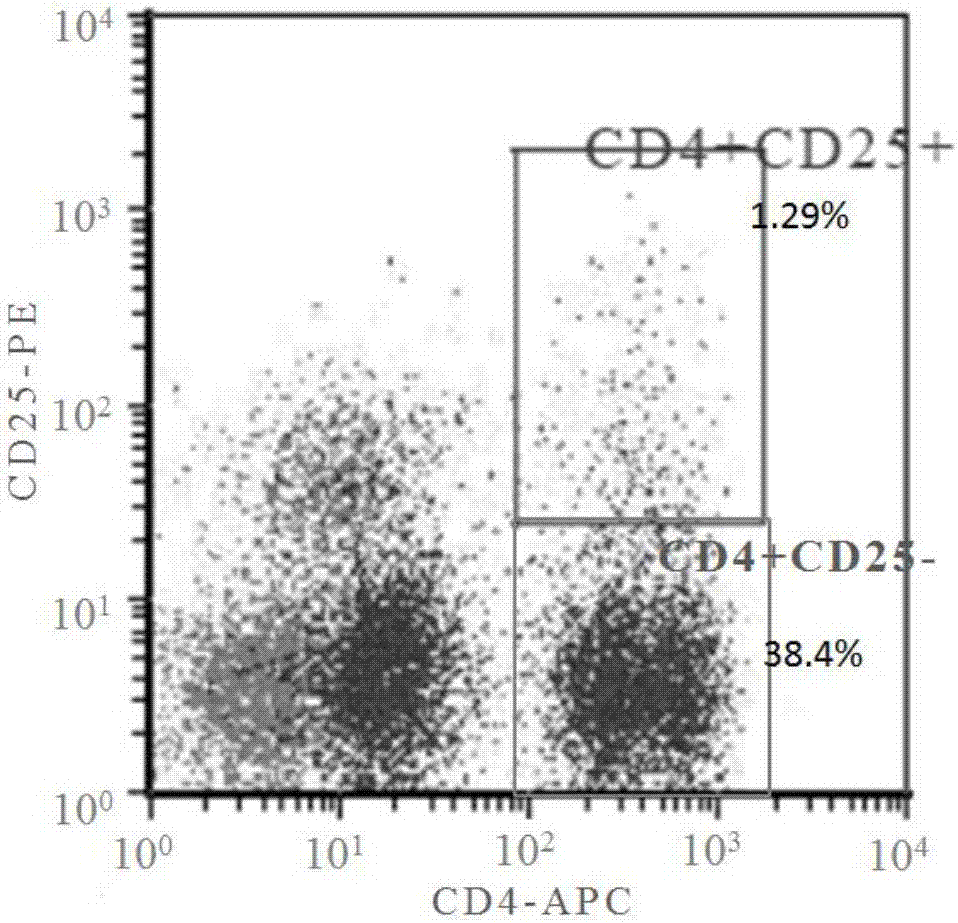
[0042] Flow cytometric detection of the cell ratio in mononuclear cells: Flow cytometry detects the ratio of CD4+CD25-T cells and CD4+CD25+Tregs in the mononuclear cells isolated in Example 3.
[0043] Collect the separated mononuclear cells, each group of 1x10^6 cells, centrifuge at 750g room temperature for 5min, discard the supernatant; 2ml PBS repeated washing cells 3 times, resuspend the cells with 100ul PBS; and add 10ul APC-CD4 and 10ul PE-CD25 antibody double staining was used as the test group, and 10ul APC-CD4 and 10ul PE-CD25 antibody single staining groups were set for fluorescence compensation, and the unstained cell group was used as the negative background. Incubate in the dark at 4°C for 30 min. After incubation, add 2ml PBS to each tube, centrifuge at 750g for 5min at room temperature, and discard the supernatant. After repeated washing with 2ml PBS for 2 times, add 200ulPBS to resuspend the cells and test on the machine.
[0044] The results showed that the rati...
Embodiment 3
[0046] An in vitro expansion method of cord blood Treg cells, the extraction method comprising the following steps;
[0047] S1: Separate mononuclear cells: Take 100ml of cord blood, centrifuge at 800g / min for 10min, collect the upper plasma, inactivate the plasma at 54°C for 40min, centrifuge at 2500rpm / min, and use it for later use; then add an equal volume of PBS to dilute the remaining blood cells , Use conventional density gradient centrifugation to separate cord blood;
[0048] S2: Adjust the cell density to 0.5x10 with RPMI1640 6 / ml, 5ml per bottle is inoculated into T25 culture flasks; inactivated plasma is added to make the final plasma concentration of 5.5%.
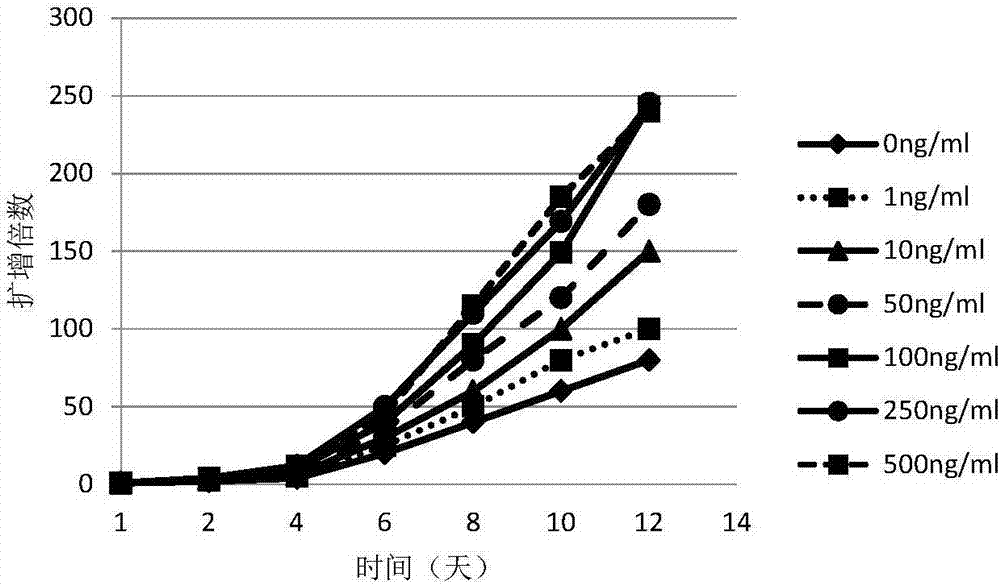
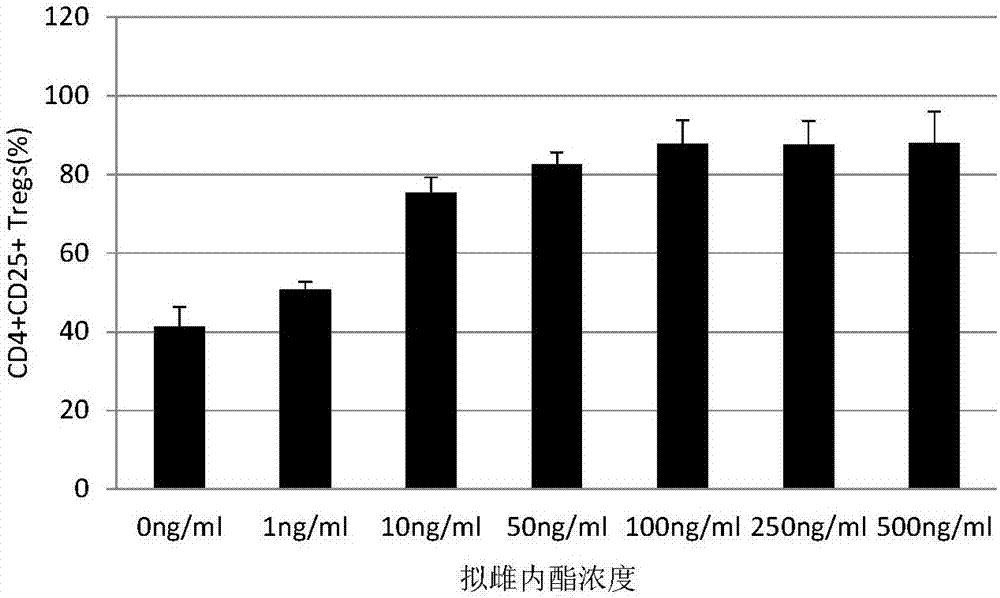
[0049] S3: The first day: add the estradiol, TGF-β, CD3 / CD28 monoclonal antibody and IL-2, and make the final concentration of each component: 100ng / ml of the estradiol, 5ng / ml of TGF-β , CD3 / CD28 monoclonal antibody 2μg / ml, IL-2 2000U / ml;
[0050] S4: Fourth day: Add 3ml RPMI1640, add inactivated plasma, estradiol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com