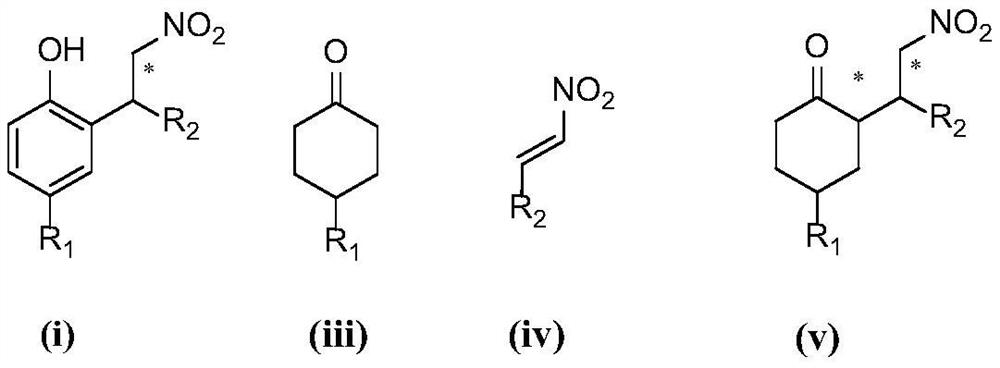
A kind of chiral synthesis method of gamma-nitrophenol compound
A synthesis method and technology for ketone compounds, which are applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of few application reports, and achieve the effects of improving reaction yield, simple operation and reducing use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the preparation of (S)-4-methyl-2-(2-nitro-1-phenethyl)phenol
[0067]
[0068] Take a clean 10mL small test tube, add 2-nitrovinylbenzene (0.6mmol, 0.0894g), 4-methylcyclohexanone (1.2mmol, 0.1344g), chiral catalyst L-proline (0.12mmol, 12.1mg ), benzoic acid (0.12mmol, 14.6mg) was dissolved in 1.0mL of dichloromethane, stirred at 25°C, after 12h, 2mL of water was added, washed with water, extracted with ether (1mL*3), added anhydrous sodium sulfate to dry, Filter and remove the solvent under reduced pressure. The crude product was separated by chromatography (petroleum ether:ethyl acetate=80:20). Michael addition product (0.5mmol), organic brominating reagent (1.0mmol, 0.2891g), copper bromide (0.075mmol, 16.8mg), benzoic acid (0.25mmol, 30.5mg), tryptophan (0.25mmol, 51.0mg), dissolved in 1mL redistilled acetonitrile solution, stirred at 100°C, detected by TLC, reacted for 12h, after the reaction, added 2mL water, washed with water, extracted with ...
Embodiment 2
[0069] Embodiment 2: Preparation of (S)-4-methyl-2-(2-nitro-1-phenethyl)phenol
[0070]
[0071] Take a clean 10mL small test tube, add 2-nitrovinylbenzene (0.6mmol, 0.0894g), 4-methylcyclohexanone (1.2mmol, 0.1344g), chiral catalyst 1,3-dimethyl-2-imidazole Linone (0.12mmol, 13.7mg), benzoic acid (0.12mmol, 14.6mg) were dissolved in 1.0mL chloroform, stirred at 10°C, after 12h, 2mL water was added, washed with water, extracted with diethyl ether (1mL*3), added Dry over anhydrous sodium sulfate, filter, and remove the solvent under reduced pressure. The crude product was separated by chromatography (petroleum ether:ethyl acetate=80:20). Michael addition product (0.5mmol), organic brominating reagent (1.0mmol, 0.2891g), copper bromide (0.075mmol, 16.8mg), benzoic acid (0.25mmol, 30.5mg), tryptophan (0.25mmol, 51.0mg), dissolved in 1mL redistilled acetonitrile solution, stirred and reacted at 100°C for 12h, after the reaction, added 2mL water, washed with water, extracted w...
Embodiment 3
[0072] Embodiment 3: Preparation of (S)-4-methyl-2-(2-nitro-1-phenethyl)phenol
[0073]
[0074] Take a clean 10mL small test tube, add 2-nitrovinylbenzene (0.6mmol, 0.0894g), 4-methylcyclohexanone (1.2mmol, 0.1344g), chiral catalyst dimercaptopyridine (0.12mmol, 13.3mg), Benzoic acid (0.12mmol, 14.6mg) was dissolved in 1.0mL ether, stirred at 40°C, after 12h, 2mL water was added, washed with water, extracted with ether (1mL*3), dried by adding anhydrous sodium sulfate, filtered, and reduced pressure Remove the solvent. The crude product was separated by chromatography (petroleum ether:ethyl acetate=80:20). Michael addition product (0.5mmol), organic brominating reagent (1.0mmol, 0.2891g), copper bromide (0.075mmol, 16.8mg), benzoic acid (0.25mmol, 30.5mg), tryptophan (0.25mmol, 51.0mg), dissolved in 1mL redistilled acetonitrile solution, stirred and reacted at 100°C for 12h, after the reaction, added 2mL water, washed with water, extracted with dichloromethane (1mL*3), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com