Fluorescent cell sensor for screening inflammasome NLRP3 activators and inhibitors
An activator and inhibitor technology, applied in the field of drug screening, can solve the problems of small processing volume, unsuitability, and inability to use NLRP3 intervention agent screening, etc., to achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Construction of NLRP3-GRE vector plasmid
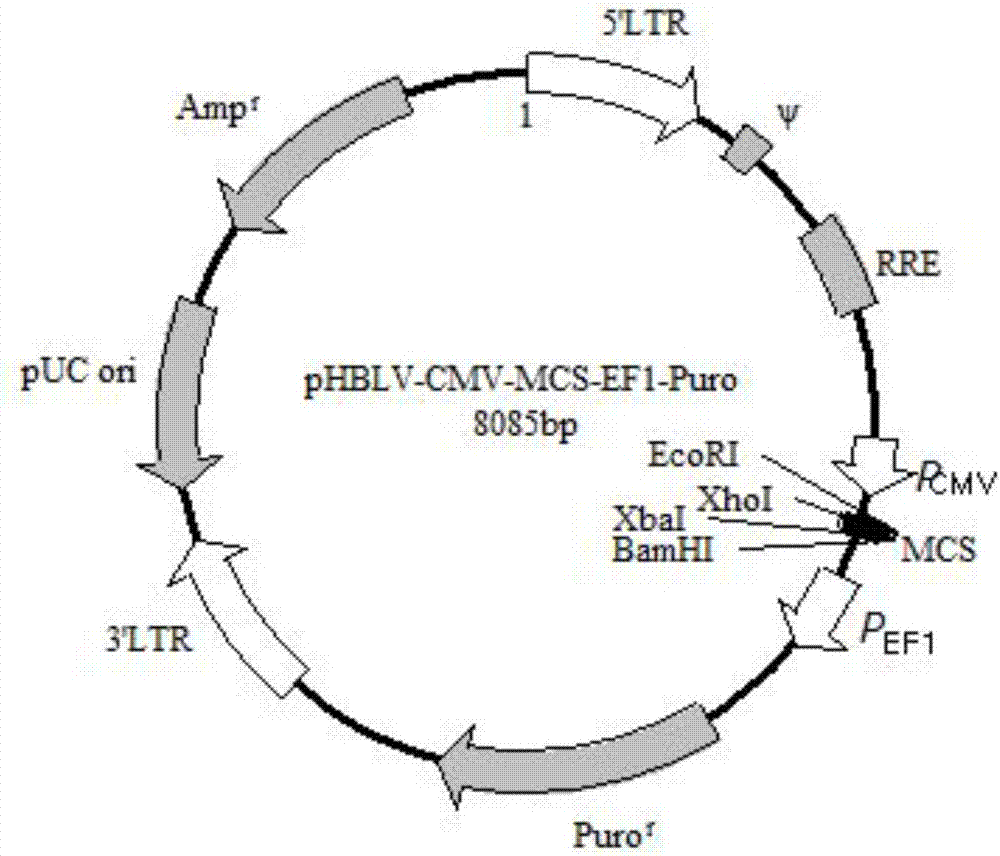
[0044] 1. The purpose is to connect the NLRP3 core promoter region with zsGREEN, and then construct pHBLV-CMVIE-EF1-Puro with ClaI / EcoRI double enzyme digestion. The vector map is as follows figure 1 shown. Since two separate sequences are spliced together to form a sequence, it is necessary to design primers in segments, and then perform a full-length PCR reaction using the PCR-derived fragments as templates. The primers were designed as follows:
[0045] NLRP3-claI-EcoRI-F: gcagagatccagtttatcgatATGCTGGGGAAGTGTGTCT
[0046] zsGREE(NNLRP3)-r:ccgtgcttggactgggccaTggtggcATGGAGGGAAAAATATGCAA
[0047] zsGREE(NNLRP3)-f:CCCTCCATgccaccAtggcccagtccaagcacgg
[0048] NLRP3-claI-EcoRI-R:agaactagtctcgaggaattcttagggcaaggcggagc
[0049] 2. The pHBLV-CMVIE-EF1-Puro vector was digested with EcoRI and ClaI. The enzyme digestion system is as follows:
[0050] 40ul enzyme digestion system (2ul 400ng / ul vector, 1ul EcoRI enzyme, 1...
Embodiment 2
[0063] Example 2 Packaging of Lentivirus Containing Plasmid NLRP3-GRE
[0064] 1. Carry out massive amplification of the vector plasmid in E. coli DH5α competent cells
[0065] (1) Take 100 μl of competent cell suspension from the -70°C refrigerator, thaw it at room temperature, and put it on ice immediately after thawing.
[0066] (2) Add 5 μg of plasmid DNA solution, shake gently, and place on ice for 30 minutes.
[0067] (3) Heat shock in a water bath at 42°C for 90 seconds. Do not move the centrifuge tube during the heat shock process. After the heat shock, quickly place it on ice to cool for 3-5 minutes.
[0068] (4) Add 1mL LB liquid culture medium (without antibiotics) to the tube, pipette and mix well, and incubate at 37°C, 220rpm shaker for 1 hour, so that the bacteria can return to normal growth state and express the antibiotic resistance encoded by the plasmid Gene.
[0069] (5) Shake the above-mentioned bacterial solution, centrifuge, remove 900 μL of supernatan...
Embodiment 3
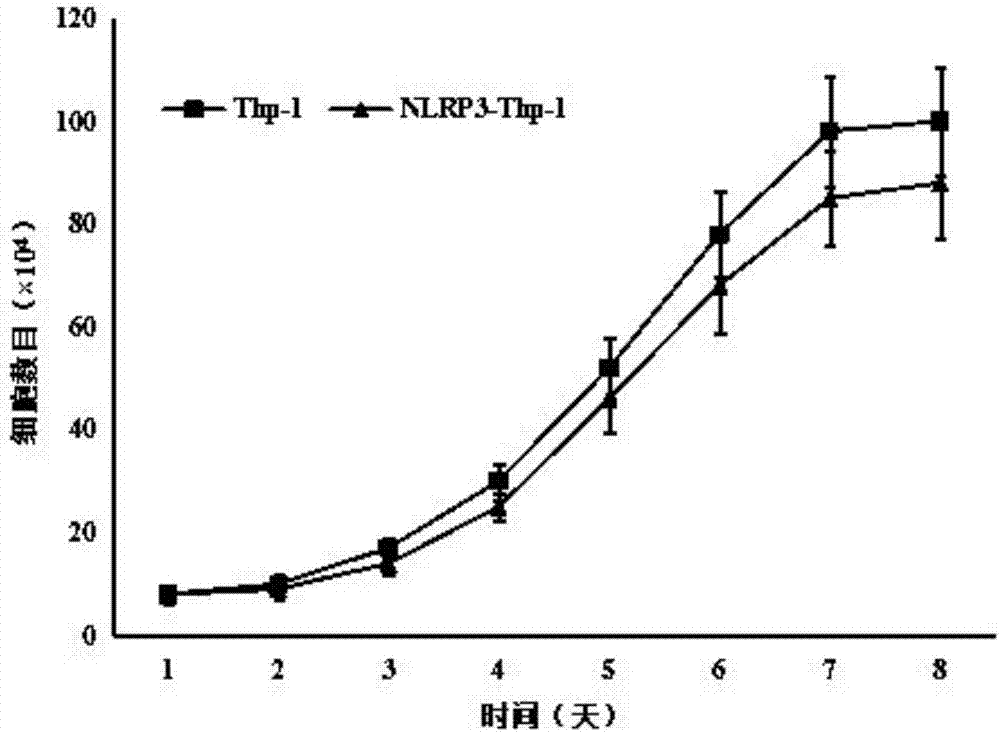
[0079] Example 3 Construction of Stably Transduced Cell Lines
[0080] 1. Infection of Thp-1 cells with lentivirus
[0081] Thp-1 cells were plated with antibiotic-free 1640+2% fetal bovine serum FBS at a cell density of 2.5×10 5 / mL, take 500 μL of cell suspension and add it to a 12-well plate, shake the 12-well plate in 8 figures to make the cells uniform; add 150 μL of lentivirus solution, and seal the 12-well plate with a parafilm
[0082] Then put it in a flat centrifuge and centrifuge at 1500rpm for 60min. After the centrifugation, remove the parafilm and place it in the incubator for 3h of infection; after 3h of infection, add 500μL of culture medium and continue for 8h of infection; 12h after infection, replace with a completely virus-free 1640 medium.
[0083] 2. Screening of stable cell lines
[0084] After 72-96 hours of infection, screening can begin. Replace the cell culture medium with complete 1640 culture medium containing 1 μg / mL puromycin, change the mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com