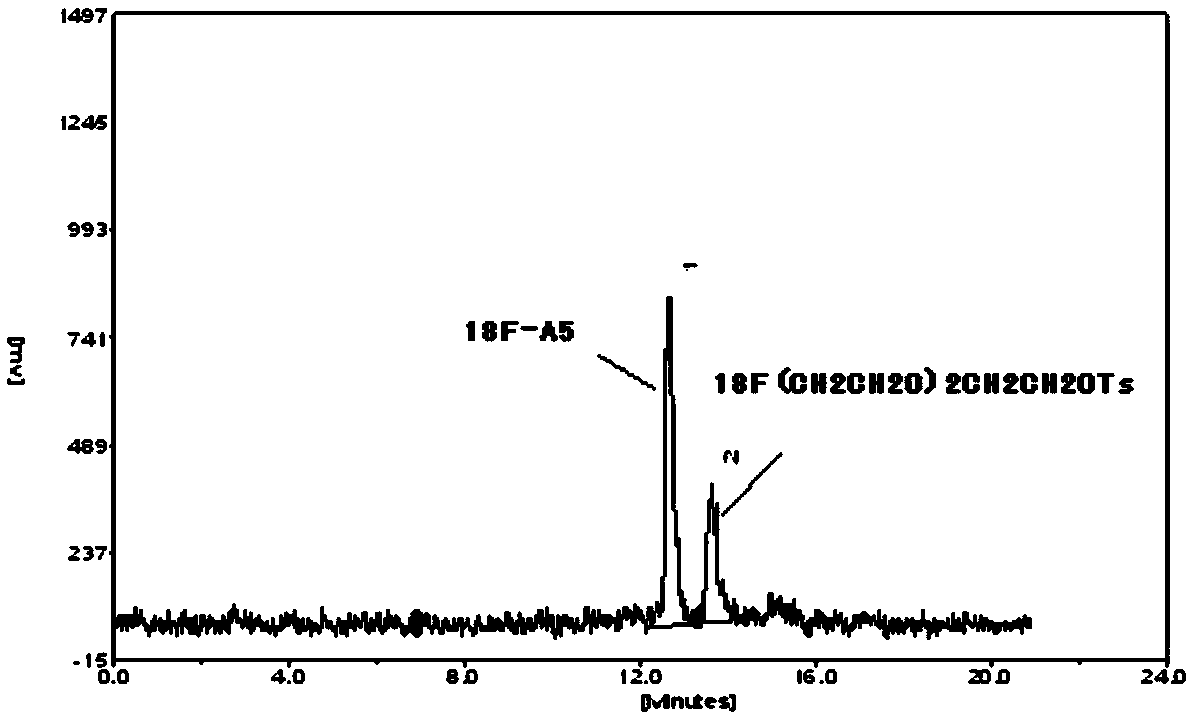
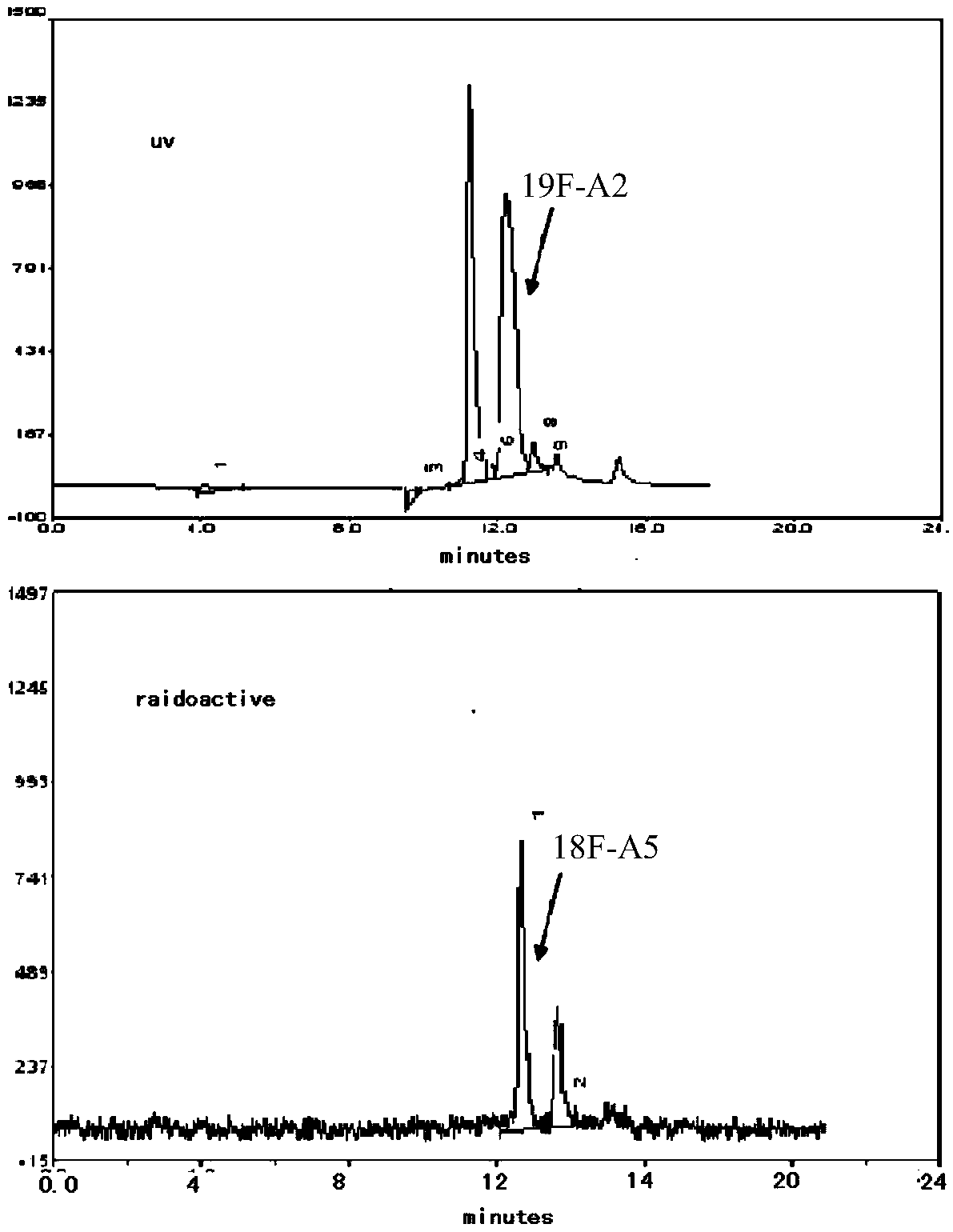
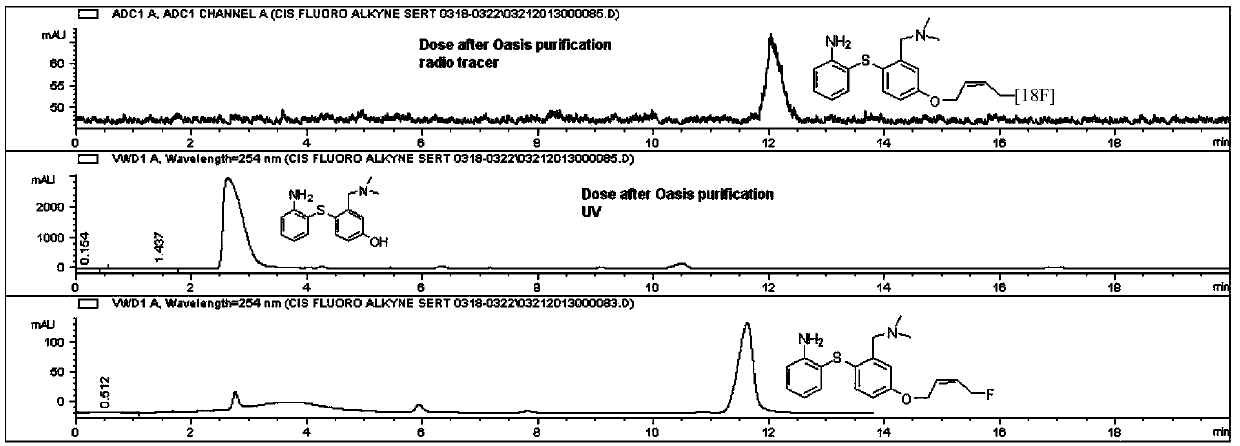
Diaryl sulfide derivatives, methods for their preparation and use as targeting imaging agents for serotonin transporters
A kind of technology of diaryl sulfide and serotonin, applied in the field of serotonin transporter targeting imaging agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of Diaryl Sulfide Derivatives
[0027] Synthesis of 2-aminobenzene-(2-(N,N-dimethyl)aminomethyl-4-(2-(2-fluoroethoxy)-ethoxy)-phenyl)sulfide (Compound A1)
[0028] Synthetic reaction equation:
[0029]
[0030] resolve resolution:
[0031] 0.15-0.2mmol 2-aminobenzene-(2-(N,N-dimethyl)methylamino-4-hydroxyl)-phenyl)sulfide (synthetic reference: (Shunichi Oya et al, Nuclear Medicine and Biology, 34(2007), 129–139) were dissolved in 4ml DMF (HPLC pure), and 2 times the molar amount of potassium carbonate and 1-p-toluenesulfonyl-2-fluoroethoxyethane (1.2 times the molar amount) were added, After passing nitrogen gas for 3-5min, the reaction solution was heated overnight in an oil bath at 80-90°C. After the reaction was completed, 30ml of water was added to terminate the reaction, and the product was extracted with ethyl acetate, and the organic phases were combined and dried using anhydrous sodium sulfate. Then it was purified by a silica gel column, and the...
Embodiment 2
[0036] Synthesis of Diaryl Sulfide Derivatives
[0037] 2-Aminobenzene-(2-(N,N-dimethyl)aminomethyl-4-(2-(2-(2-fluoroethoxy)-ethoxy)-ethoxy)-benzene) Synthesis of Thioether (Compound A2)
[0038] Synthetic reaction equation:
[0039]
[0040] Dissolve 0.15-0.2mmol 2-aminobenzene-(2-(N,N-dimethyl)aminomethyl-4-hydroxy)-benzene)sulfide in 4ml DMF (HPLC pure), add 2 times molar amount Potassium carbonate and 1-p-toluenesulfonyl-(2-(2-fluoroethoxy)-ethoxy)ethane (1.2 times the molar weight), then pass into nitrogen for 3-5min, and react the solution at 80- Heat in an oil bath at 90°C overnight. After the reaction, 30ml of water was added to terminate the reaction, and the product was extracted with ethyl acetate, the combined organic phases were mixed and dried using anhydrous sodium sulfate, then purified by a silica gel column, and the eluent used was methanol: dichloromethane: diethylamine =4:100:1 (volume ratio), the target product compound A2 was obtained, yield: 22.6%...
Embodiment 3
[0045] Synthesis of Diaryl Sulfide Derivatives
[0046] Synthesis of 2-aminobenzene-(2-(N,N-dimethyl)aminomethyl-4-(cis-4-fluoro-2-enebutoxy)-benzene)sulfide (compound A3)
[0047] Synthetic reaction equation:
[0048]
[0049] resolve resolution:
[0050] ① Preparation of 1,4-di-p-toluenesulfonyl-2-butene
[0051] Dissolve 0.3ml of cis-2-butene-1,4-diol (3.16mmol) in 15ml of freshly distilled tetrahydrofuran (THF). After stirring in the bath for 10 min, 1 g of sodium tert-butoxide was added to obtain a white milky product, and the stirring was continued overnight. After the reaction, remove the solvent with a rotary evaporator, add 20ml of water, and extract three times with dichloromethane, combine the organic phases, and purify by column chromatography after drying over anhydrous sodium sulfate, the eluent is ethyl acetate:petroleum ether= 1:4 (volume ratio) to obtain 1,4-di-p-toluenesulfonyl-2-butene. Yield: 12.2%.
[0052] ② Preparation of 1-fluoro-4-p-toluenesul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com