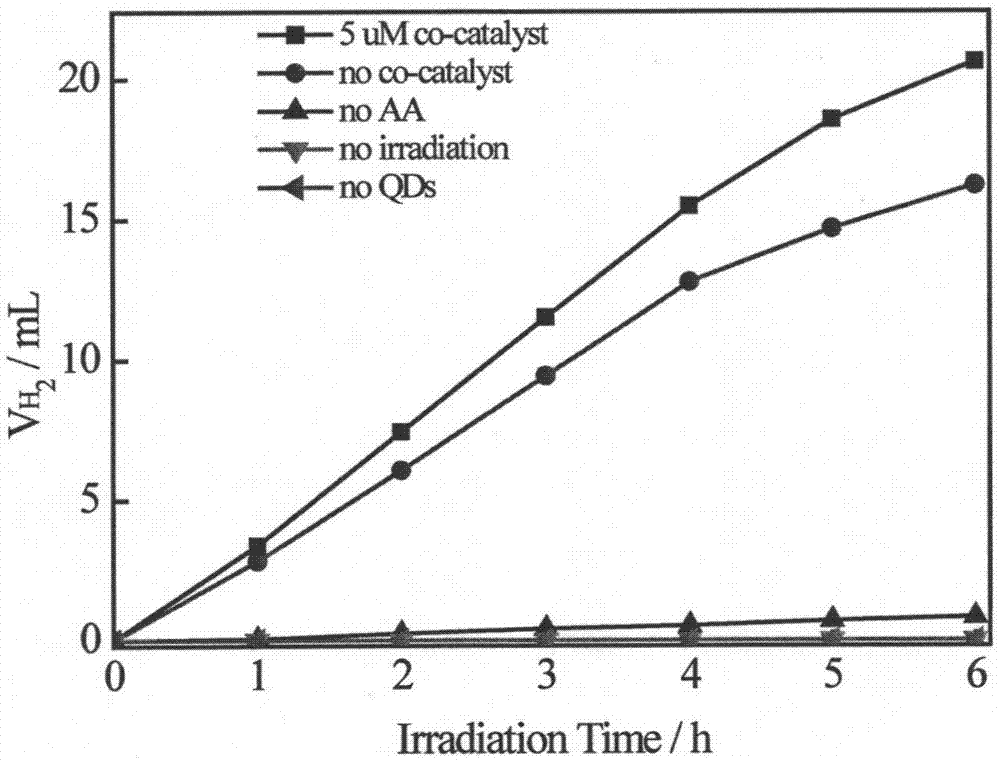
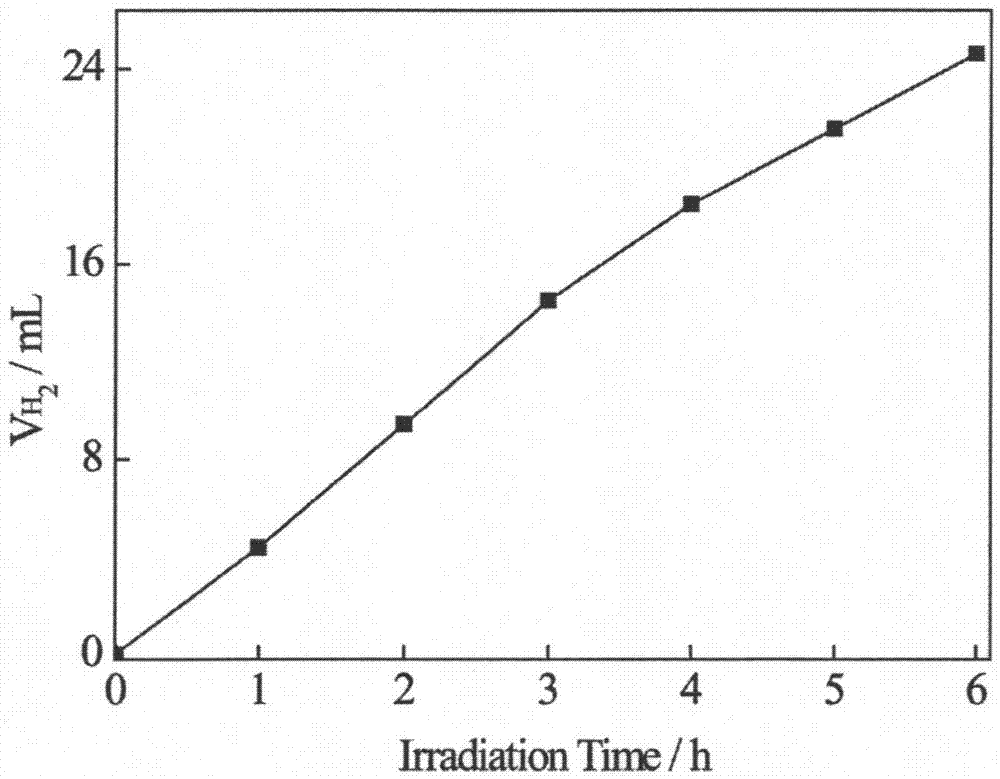
CuInS2 quantum dots, CuInS2/ZnS quantum dots and their preparation and application
A technology of quantum dots and compounds, which is applied in the field of CuInS2 and CuInS2-ZnS quantum dots, can solve the problems of oil-phase quantum dots and water-phase quantum dots, and achieve high-efficiency photolysis of water to produce hydrogen, simple process, and good visible light absorption properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] CuInS 2 Preparation method of quantum dots:
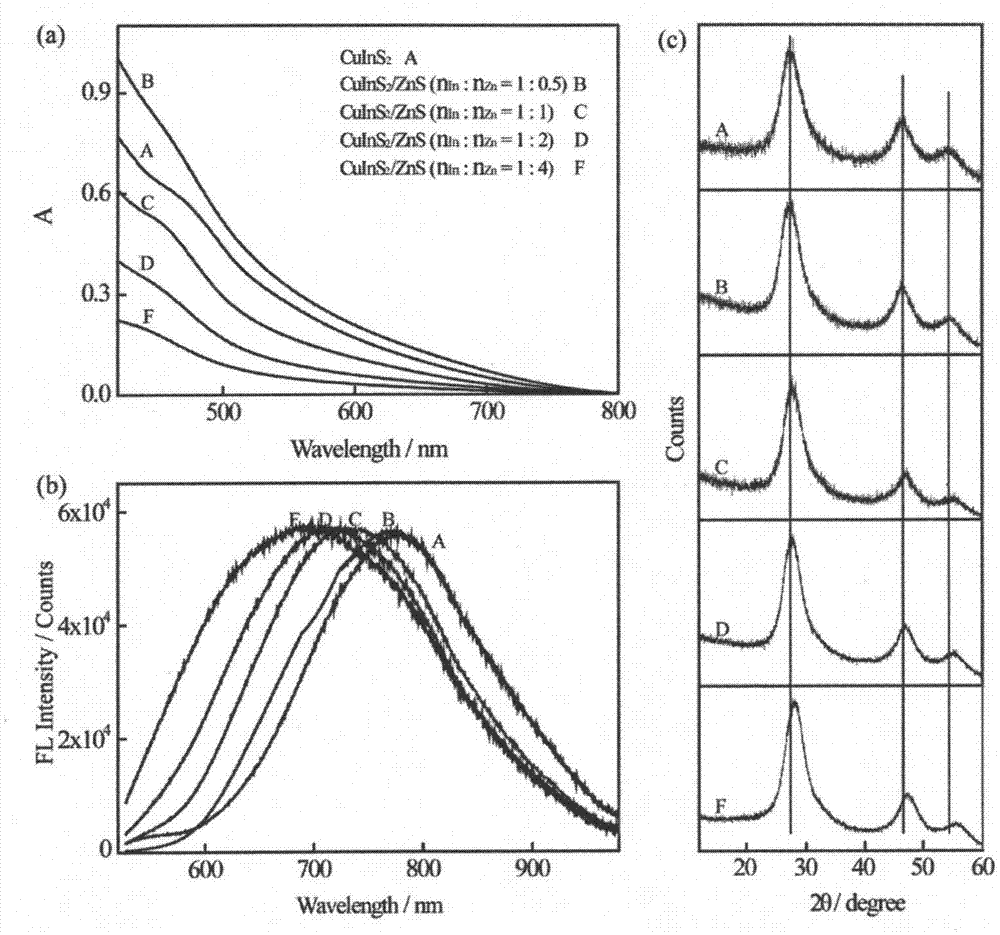
[0047] Weigh 85.0 mg (0.5 mmol) of copper chloride dihydrate, 586.0 mg (2.0 mmol) of indium trichloride tetrahydrate, and 5.97 g (20.0 mmol) of glutathione into a 500 mL three-necked flask containing 125 mL of deionized water , Adjust the pH to 8.5 with a 0.2M aqueous sodium hydroxide solution to obtain mixed solution A; then add sodium sulfide (0.31g, 4.0mmol) dissolved in 10mL of water to obtain mixed solution B; warm up mixed solution B to 100℃ and Stir vigorously at this temperature at 1500 rpm for 4.0 hours and then cool to room temperature; add 125 mL of ethanol to the mixture, and centrifuge to separate a black solid. After washing twice with ethanol, it is dried in a vacuum oven at 40°C to obtain orange yellow Solid, namely CuInS 2 Quantum dots. Such as figure 1 For the prepared CuInS 2 Ultraviolet-visible absorption, fluorescence spectrum and X-ray diffraction spectrum of quantum dots.
Embodiment 2
[0049] CuInS 2 / ZnS quantum dot preparation (n In : N Zn =1:0.5)
[0050] Weigh 85.0 mg (0.5 mmol) of copper chloride dihydrate, 586.0 mg (2.0 mmol) of indium trichloride tetrahydrate, and 5.97 g (20.0 mmol) of glutathione into a 500 mL three-necked flask containing 125 mL of deionized water , Adjust the pH to 8.5 with a 0.2M aqueous sodium hydroxide solution to obtain mixed solution A; then add sodium sulfide (0.31g, 4.0mmol) dissolved in 10mL of water to obtain mixed solution B; heat the resulting 135mL mixed solution B to 100°C and stirring vigorously at 1500rpm at this temperature for 4.0 hours, add 214.5mg (1.0mmol) zinc acetate dihydrate, 76.1mg (1.0mmol) thiourea and 461.0mg (1.5mmol) of glutathione. 20mL of an aqueous solution with a pH of 12.5, mixed at 100℃ and continued to stir vigorously at 1500rpm for 45 minutes, then cooled to room temperature, 200mL of ethanol was added, and a dark brown solid was obtained by centrifugation. After washing twice with ethanol, vac...
Embodiment 3
[0052] CuInS 2 / ZnS quantum dot preparation (n In : N Zn = 1:1)
[0053] Weigh 85.0 mg (0.5 mmol) of copper chloride dihydrate, 586.0 mg (2.0 mmol) of indium trichloride tetrahydrate, and 5.97 g (20.0 mmol) of glutathione into a 500 mL three-necked flask containing 125 mL of deionized water , Adjust the pH to 8.5 with a 0.2M aqueous sodium hydroxide solution to obtain mixed solution A, then add sodium sulfide (0.31g, 4.0mmol) dissolved in 10mL of water to obtain mixed solution B; heat the resulting 135mL mixed solution B to 100°C and vigorously stirred at 1500rpm at this temperature for 4.0 hours. After adding 429.0mg (2.0mmol) zinc acetate dihydrate, 152.2mg (2.0mmol) thiourea and 922.0mg (3.0mmol) glutathione 20mL of an aqueous solution with a pH of 12.5, mixed at 100℃ and continued to stir vigorously at 1500rpm for 45 minutes, then cooled to room temperature, 200mL of ethanol was added, and a dark brown solid was obtained by centrifugation. After washing twice with ethanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com