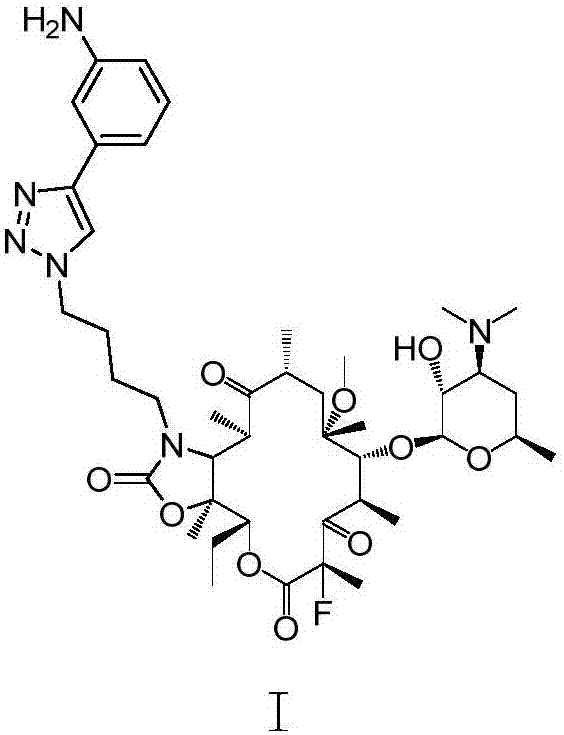
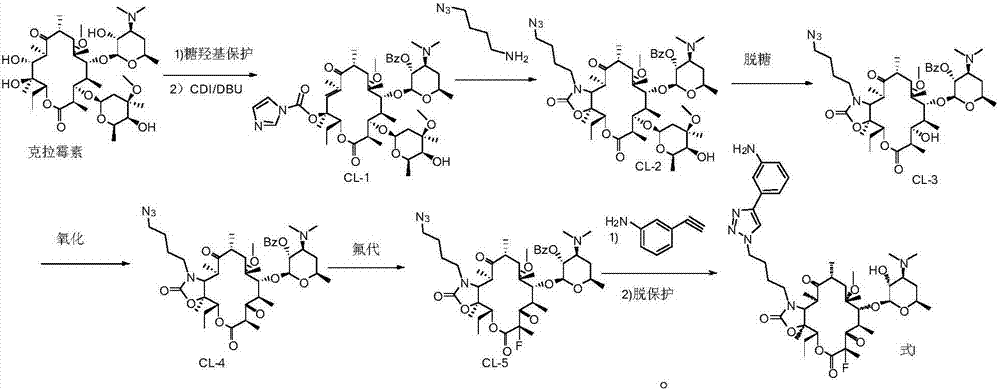
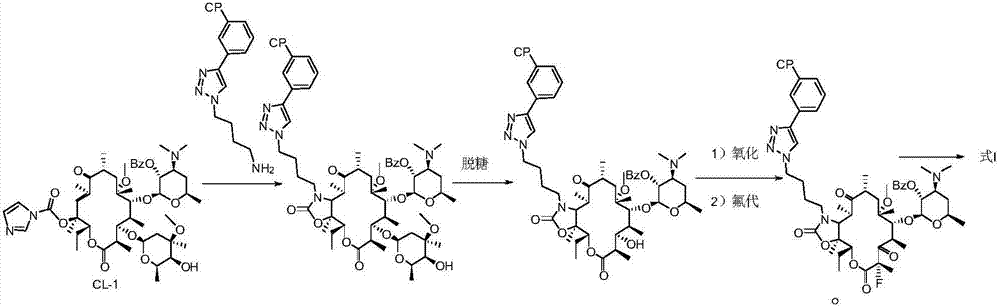
Preparation method of solithromycin
A technology of solithromycin and its compound, which is applied in the field of preparation of macrolide drug solithromycin, and can solve the problems that it is not suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Preparation of Intermediate II:
[0074] ⑴Preparation of Compound A
[0075]
[0076] Add clarithromycin (50 g, 0.067 mol), triethylamine (18.75 mL, 0.135 mol, 2 equivalents), and ethyl acetate (350 mL) into a 500 mL reaction flask and stir to mix. Benzoic anhydride (22.5 g, 0.1 mol, 1.5 equiv) was added in portions. After the addition, stir at room temperature (20-25°C) for 24h. After detecting that the clarithromycin reaction was complete, the solvent was distilled off under reduced pressure (temperature<45° C.). Add 500 mL ice methanol to the residue, stir in ice bath (0-5° C.) for 0.5 h, and filter with suction. The filter cake was rinsed with ice methanol (100 mL×2) and dried in vacuo to obtain 56 g of Compound A as a white solid.
[0077] ESI[M+1]:852
[0078]
[0079] Add compound A (56 g, 0.0657 mol), ethanol (300 mL), and water (300 mL) obtained in the previous step into a 1000 mL reaction flask and mix and stir. Concentrated hydrochloric acid (56 m...
Embodiment 2
[0107] Preparation of compound Ⅲ by fluorination reaction
[0108]
[0109] Dissolve 2.05 g of acetyl-protected intermediate II (2.90 mmol) in a mixed solution of 20 ml of DMF / THF (tetrahydrofuran) (9:1), and add 0.39 g of potassium tert-butoxide (3.48 mmol), the addition was completed, the mixture was stirred at -20°C for 0.5 hours, then 1.01 g of NFSI (N-fluorobisbenzenesulfonamide) (3.19 mmol) was added, the addition was completed, and the reaction was continued at -20°C for two hours. Sampling HPLC detects that the reaction is complete, adding a small amount of water to quench the reaction, diluting the reaction solution with 50 milliliters of ethyl acetate, then washing (6*50 milliliters) with saturated brine several times, drying over anhydrous sodium sulfate, filtering and spinning to obtain the crude product ( can be used directly in the next step). The crude product was purified by column chromatography (silica gel 200-300 mesh, Qingdao Huanghai), using 4 L of eth...
Embodiment 8
[0119] Preparation of compound III
[0120]
[0121] Compound VII (6.74 g, 0.01 mol) was dissolved in DMF:THF=9:1 mixed solvent (40 ml) and replaced with nitrogen. The reaction solution was cooled to -25°C. Sodium hydrogen (1 g, 0.025 mol, 2.5 eq) was added. After the addition is complete, react at -20°C to -25°C for 1h. NFSI (5 g, 0.015 mol, 1.5 eq) was added in portions. After the addition is complete, react at -20°C to -25°C for 1h. After the reaction of the raw materials was detected by sampling, 90ml of ice water was added dropwise, and the temperature was controlled not to exceed 10°C, and solids were precipitated. After dropping, stir for 15 minutes, filter with suction, rinse with ice water. The filter cake was dissolved in 50 ml of ethyl acetate, washed with saturated brine, and dried over anhydrous sodium sulfate. Suction filtration and spin-drying gave 6.67 g of a light yellow solid crude product, MS=692. The crude product was directly reacted in the next ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com