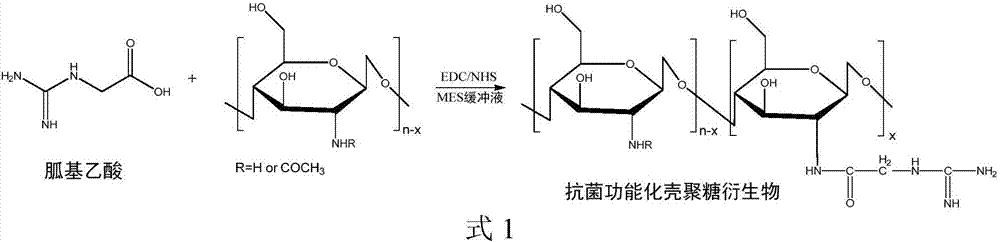
Preparation method of antimicrobial functionalized chitosan derivative
A technology for chitosan derivatives and chitosan transformation is applied in the field of preparation of antibacterial functionalized chitosan derivatives, which can solve the problems of insufficient water solubility of chitosan, antibacterial properties, influence, and low substitution degree. , to achieve the effect of improving antibacterial and solubility, high biosafety, and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 0.1 gram of chitosan and add it to 100 milliliters of 0.1M dilute hydrochloric acid, and mechanically stir at room temperature to dissolve the chitosan completely, thereby obtaining a 0.1% chitosan dilute acid solution by mass; A mixed solution of guanidinoacetic acid, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) for 3 hours ( The solvent is a buffer solution of 10mM 2-(N-morpholino)ethanesulfonic acid (MES), pH=4.5) 20mL is added in the above-mentioned chitosan dilute acid solution, wherein chitosan, guanidinoacetic acid, NHS, EDC The ratio of the amount of the substance is 10:1:5:5, after 12 hours of continuous stirring reaction at 35 ° C, add the hydroxylamine hydrochloride with the amount of guanidinoacetic acid and other substances to terminate the reaction; filter the insoluble matter in the reaction solution, and then Transfer the filtrate into a dialysis bag, seal both ends of the dialysis bag tightly...
Embodiment 2
[0034] Take by weighing 1.0 gram of chitosan and join in the dilute hydrochloric acid of 100 milliliters 0.2M, mechanically stir at room temperature so that chitosan dissolves completely, thereby obtain the chitosan dilute acid solution that mass percentage is 1.0%; A mixed solution of guanidinoacetic acid, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) activated for 2 hours (the solvent is the buffer solution of 2-(N-morpholino)ethanesulfonic acid (MES) of 25mM, pH=5.0) 35mL is added in above-mentioned chitosan dilute acid solution, wherein chitosan, guanidinoacetic acid, NHS, The ratio of the amount of substances in EDC is 2:2:5:5, after 24 hours of continuous stirring reaction at room temperature, add hydroxylamine hydrochloride with the amount of guanidinoacetic acid and other substances to terminate the reaction; filter the insoluble matter in the reaction solution, and then Transfer the filtrate to a dialysis bag, sea...
Embodiment 3
[0036]Take by weighing 10.0 gram chitosan and join in the dilute hydrochloric acid of 100 milliliters 0.3M, under room temperature mechanical stirring makes chitosan dissolve completely, thereby obtain the chitosan dilute acid solution that mass volume percent concentration is 9.1%; A mixture of guanidinoacetic acid, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) activated for 1 hour Solution (solvent is 50mM buffer solution of 2-(N-morpholino)ethanesulfonic acid (MES), pH=6.5) 20mL is added in above-mentioned chitosan dilute acid solution, wherein chitosan, guanidinoacetic acid, NHS , The ratio of the amount of EDC to the substance is 1:5:5:5, after 48 hours of continuous stirring reaction at 15°C, add hydroxylamine hydrochloride with the amount of guanidinoacetic acid and other substances to terminate the reaction; filter to remove insoluble matter in the reaction solution , then transfer the filtrate into a dialysis bag,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com