PD-1 targeted polypeptide and application thereof
A technology of PD-1 and PD-L1, which is applied to peptides targeting human PD-1 protein and its application fields, and can solve the problems of easy immunogenicity, poor tissue permeability, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
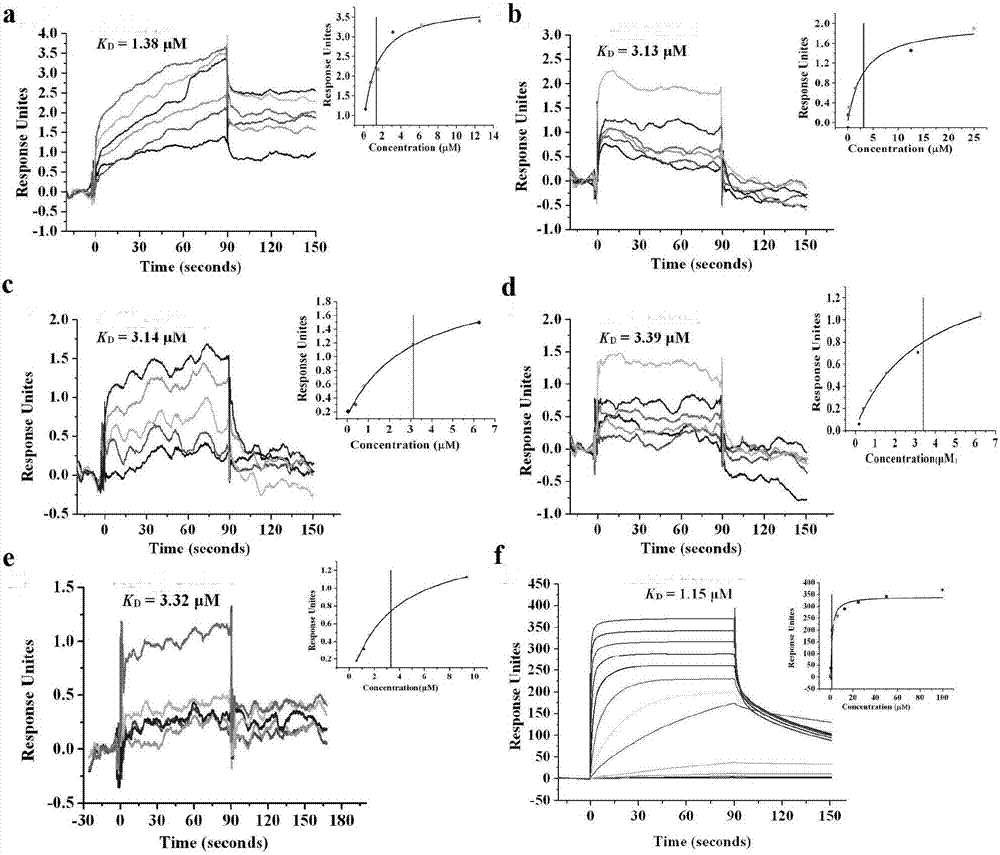
Examples
Embodiment 1
[0084] Embodiment 1. The synthesis of polypeptide of the present invention
[0085] (1) Experimental instruments and materials
[0086] Dimethylformamide (DMF), piperidine, resin, dichloromethane (DCM), ninhydrin reagent, benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate salt (HBTU), triisopropylsilane (TIS), ethanedithiol (EDT), anhydrous ether, trifluoroacetic acid (TFA), N-methylmorpholine (NMM), methanol, ethanol, 20 kinds of amino acids , peptide solid-phase synthesis tube, mass spectrometry instrument MicrOTOF-Q11 (BrukerDaltonics).
[0087] (2) Experimental steps
[0088] Weigh the resin and put it into the peptide solid-phase synthesis tube, add an appropriate amount of DMF to swell for more than half an hour. The DMF was removed, and the Fmoc deprotection reaction was carried out with a deprotection solution (piperidine:DMF=1:4), and placed on a shaking table for 10 minutes. Remove the deprotection solution, wash three times with DMF and DCM, take a s...
Embodiment 3
[0089] Example 3. Construction of Human PD-1 Recombinant Plasmid
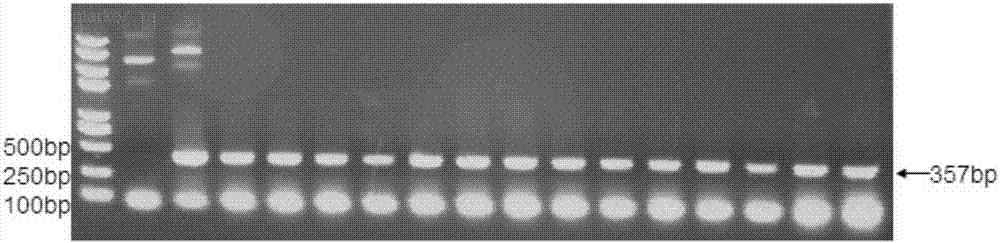
[0090] The target gene is the coding nucleic acid of amino acids 34-150 on the PD-1 protein. The target fragment was cloned into the pET-28a vector (Novagen Company) using the specific primers designed by NcoI and NdeI restriction sites. The forward primer is: 5'-TTTTCCATGGGTCCCCCCCACCTTCTCCCCAG-3' (SEQ ID NO: 10); the reverse primer is: 5'-GCCGCCGCATATGTTATTCTGCCCTTCTCTCTG-3' (SEQ ID NO: 11). The human PD-1 gene was used as a template for PCR amplification. The PCR system is: 2 μL PD-1 plasmid, 2 μL forward primer, 2 μL reverse primer, 5 μL 10× buffer solution, 4 μL dNTP, 1 μL pfu polymerase, 34 μL ddH 2 O. PCR amplification conditions: pre-denaturation at 94°C for 4 min; denaturation at 94°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 48 s, 30 cycles; 10 min at 72°C. After the PCR amplification is completed, perform nucleic acid electrophoresis, cut out the target band, and recover the PCR...
Embodiment 4
[0091] Example 4. Expression and purification of human PD-1 protein
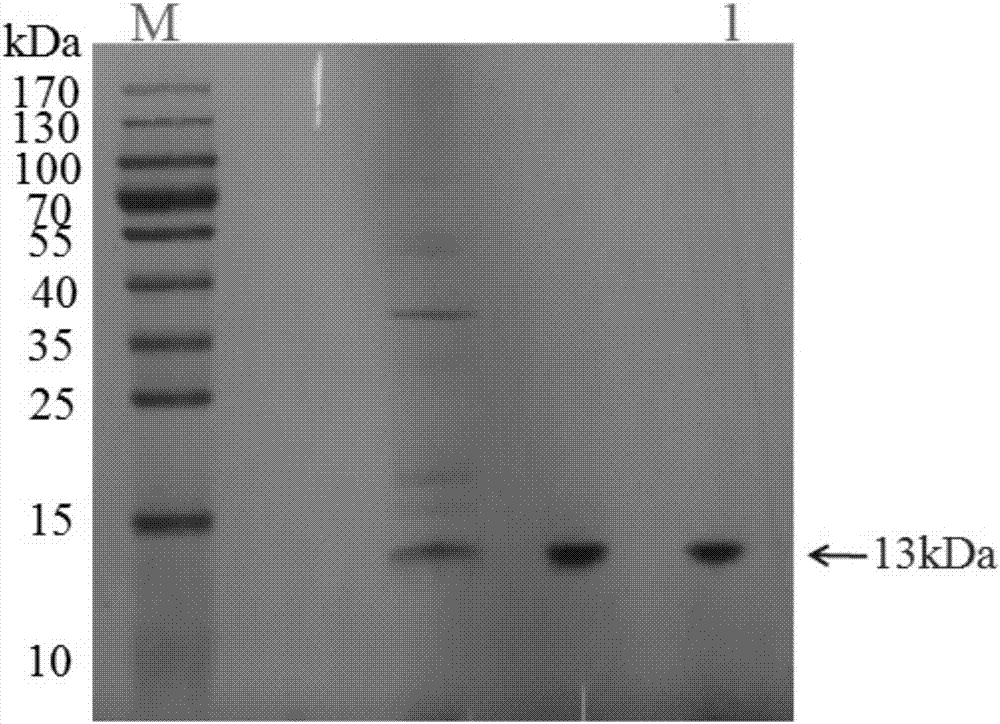
[0092] The human PD-1 protein used in the present invention is expressed and purified by prokaryotes. The specific experimental steps are as follows: the recombinant plasmid with correct sequencing was transformed into E.coli BL21 competent cells to induce expression. A single colony was picked and inoculated in TB medium containing kanamycin, and cultured overnight at 37°C with shaking. The next day, expand the small cultured product into 1L of TB medium and culture to OD 600 0.5-0.6, add the inducer IPTG to a final concentration of 0.5mM, and induce at 37°C for 5-7h. Harvest bacteria at 4,000rpm, first lyse the bacteria with lysis buffer solution (50mM Tris-HCl, pH 8.0, 50mM NaCl, 1mM DTT, 0.5mMEDTA, 5% glycerol), then high-pressure crush, and centrifuge at 12,000rpm to get the precipitate . Wash twice with washing buffer (20 mM Tris-HCl, pH 8.0, 2M urea, 2.5% Triton X-100), and collect the precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com