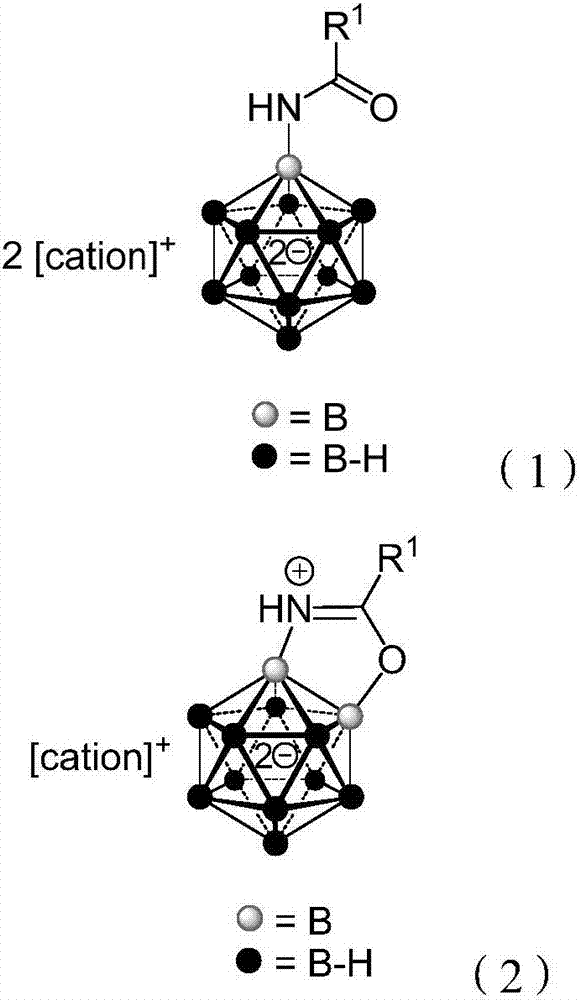
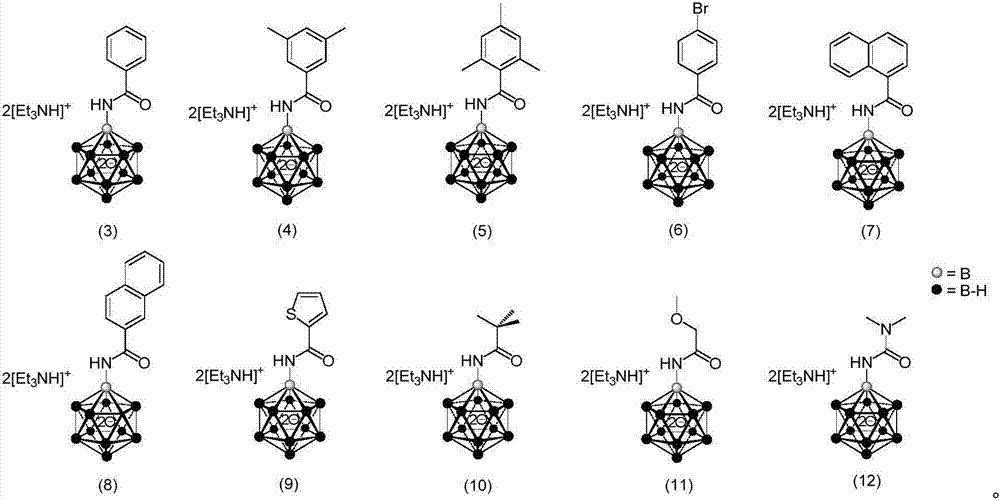
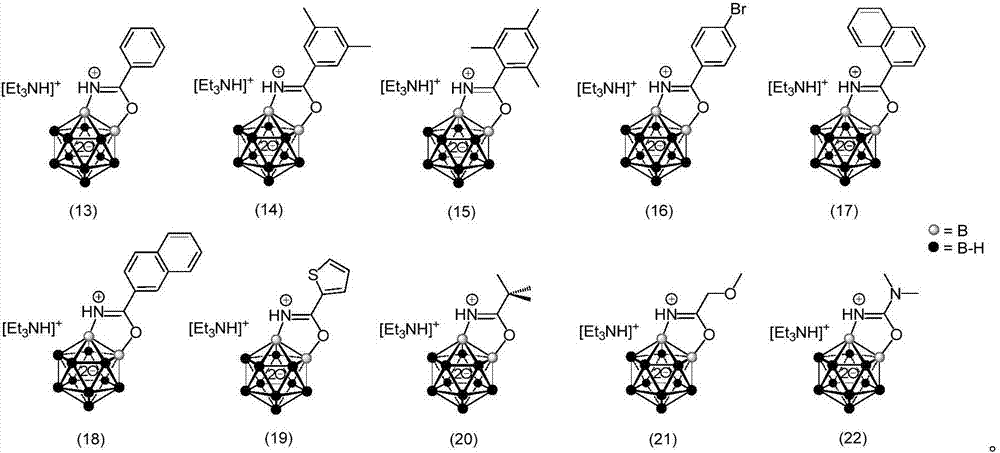
Borane compound used for antibacterial treatment and preparation method of borane compound
A borane compound and compound technology, applied in the field of borane compound and its preparation, can solve limitations and other problems, and achieve the effects of short synthesis path, mild reaction conditions, and susceptibility to drug-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Under nitrogen protection, add 6 mL of anhydrous THF to a 20 mL reaction flask containing monoaminated dodecaborane (300 mg, 1.1 mmol) and NaH (90 mg, 3.7 mmol) of the structure shown in formula (23), and stir at room temperature for half hours, the solution stopped bubbling. Benzoyl chloride (177 mg, 1.3 mmol) was then added slowly over half an hour. Then stir at room temperature for half an hour. After the reaction was completed, it was quenched with water and diluted, followed by the addition of Et 3 NHCl (303mg, 2.2mol), fully stirred to make the cation exchange complete, the resulting mixture was extracted 7 times with a mixture of dichloromethane and acetonitrile with a volume ratio of 4:1, and the resulting organic phase was extracted with MgSO 4 dry. Subsequent filtration, the solvent of the obtained filtrate was removed by rotary evaporation, and the residue was subjected to silica gel column chromatography (dichloromethane / acetonitrile=4:3) to o...
Embodiment 2
[0049]
[0050] The synthetic method of the compound shown in formula (4) is the same as in Example 1, the only difference is that the acid chloride used is 3,5-dimethylbenzoyl chloride (219mg, 1.3mmol), the product obtained is a white solid, and the yield : 85%; 1 H{ 11 B}NMR (400MHz, CD 3 CN): δ7.28(s,2H,phenyl H),7.09(s,1H,phenyl H),6.21(s,1H,anionic N-H),3.18(q,J=7.2Hz,12H,cationic N-CH 2 ),2.31(s,6H,methyl H),1.47-0.79(broadoverlapping m,11H,B-H),1.25(t,J=7.4Hz,18H,N-CH 2 CH 3 ); 13 C{ 1 H}NMR (100MHz, CD 3 CN): δ170.0 (C=O), 139.0, 138.9, 132.6, 125.3 (4phenyl signals), 47.8 (cationic signal), 21.2 (methyl signal), 9.1 (cationic signals); 11 B{ 1 H}NMR (128MHz, CD 3 CN): δ-5.1(1B,B-N),-15.3(5B,B-H),-16.5(5B,B-H),-18.8(1B,B-H).HRMS(ESI) calculated for C 9 h 22 B 12 NO - : 290.2891, Found: 290.2864.
Embodiment 3
[0052]
[0053] The synthetic method of the compound shown in formula (5) is the same as in Example 1, the only difference is that the acid chloride used is 2,4,6-trimethylbenzoyl chloride (237mg, 1.3mmol), and the product obtained is a white solid, Yield: 77%; 1 H { 11 B}NMR (400MHz, CD 3 CN):δ7.81(br,2H,cationic N-H),6.84(s,1.4H,phenyl H of majorisomer),6.74(s,0.6H,phenyl H of minor isomer),3.09(q,J=7.4Hz ,12H,cationic N-CH 2 ),2.24(s,3H,para CH 3 ),2.21(s,6H,ortho CH 3 ),1.47-0.67(broad overlapping m,11H,B-H),1.21(t,J=7.4Hz,18H,cationic N-CH 2 CH 3 ); 13 C{ 1 H}NMR (100MHz, CD 3 CN): δ176.1(minor isomer of C=O), 173.4(major isomer of C=O), 139.3(minor isomer of paraphenyl carbon), 137.8(major isomer of paraphenyl carbon), 136.1(C ipso ),135.3(minor isomer of ortho phenyl carbon),134.9(major isomer of ortho phenylcarbon),47.7(cationic signal),21.2(minor isomer of para CH 3 ), 21.1 (majorisomer of para CH 3 ),20.2(minor isomer of ortho CH 3 ), 19.2 (major isomer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com