Novel polypeptide PAP1 and application thereof
A technology for new peptides and supplies, applied in the new peptide PAP1 and its application fields, can solve the problems of high preparation cost and poor stability, and achieve the effects of promoting migration, easy synthesis, and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of new polypeptide PAP1
[0029] Using the conventional solid-phase synthesis method of an automatic peptide synthesizer, the synthesis of the new peptide PAP1 was completed through resin swelling, deprotection, washing, amino acid dissolution, amino acid activation, and condensation (entrusted to Hefei Guopeptide Biotechnology Co., Ltd. to synthesize).
Embodiment 2
[0030] Example 2 Structural Identification of Polypeptide PAP1
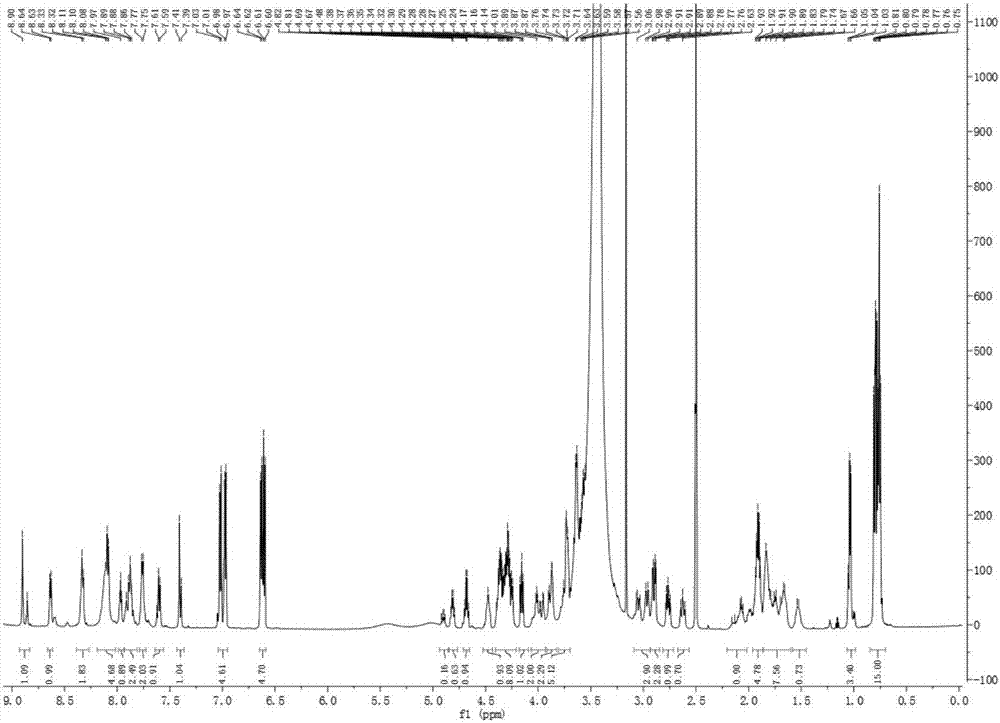
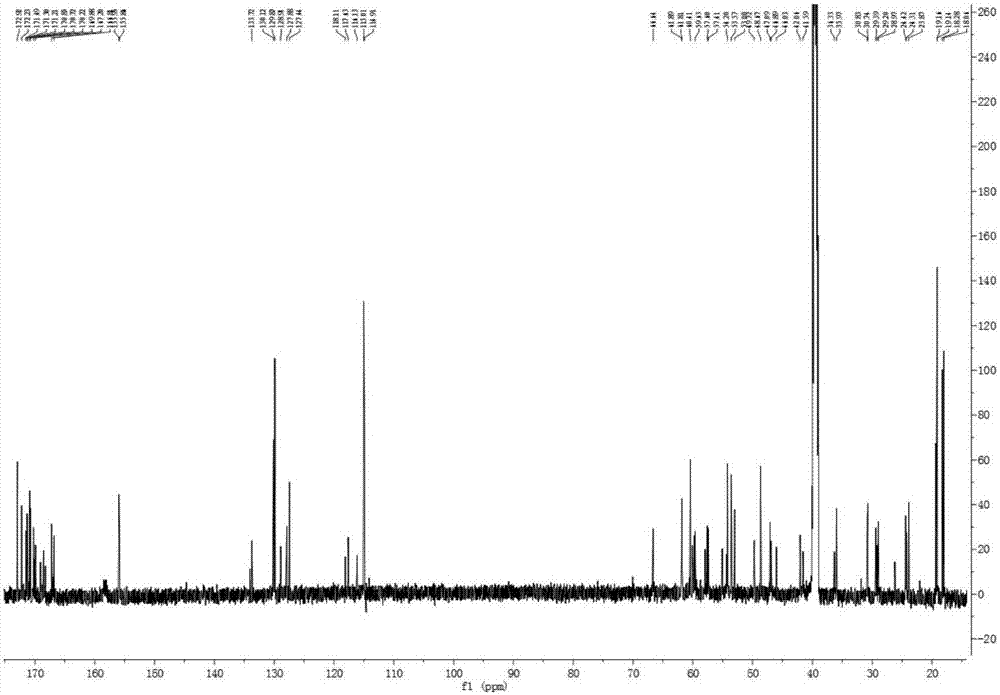
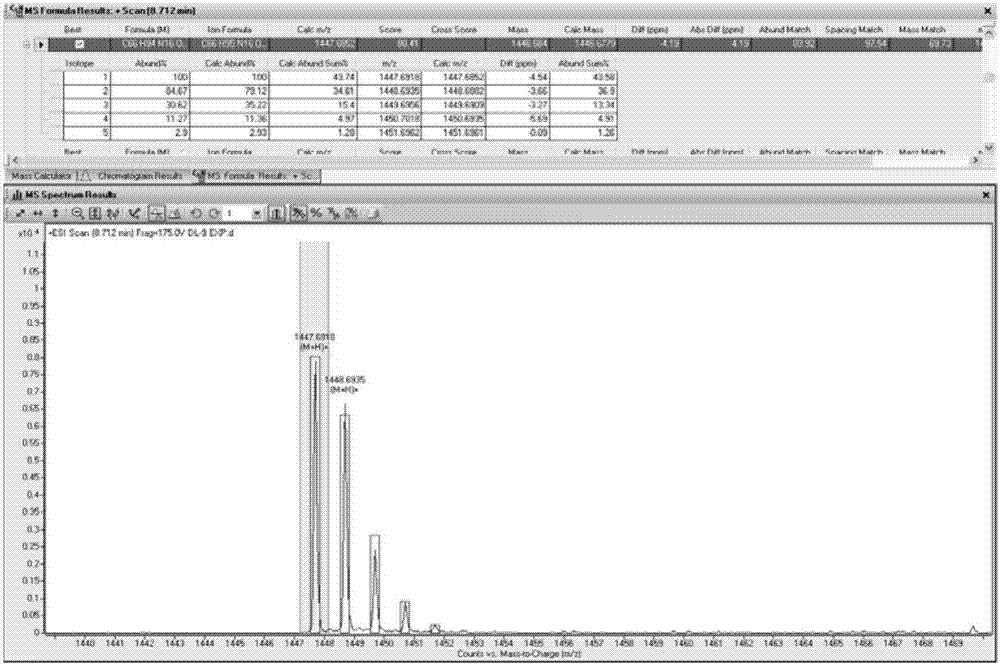
[0031] The new polypeptide PAP1 that embodiment 1 makes is white powder; UV (MeOH) λ max (logε)205(3.85),224(1.61),279(0.12)nm; IR(KBr)ν max 3300, 2968, 2889, 1664, 1537, 1447, 1205, 1132, 801, 722cm -1 ;HR-ESI-MS m / z 1447.6919[M+H] + (C 66 h 94 N 16 o 21 , calculated value 1447.6852). Hydrogen spectrum ( 1 H NMR) and carbon spectrum ( 13 C NMR) data in Table 1 (assigned by 2D-NMR). The amino acid sequencing result was: H-Ser-Ser-Pro-Tyr-Thr-Ser-Gly-Pro-His-Pro-Gly-Val-Val-Tyr-OH. That 1 H, 13 C NMR spectrum see Figure 1~2 ; high-resolution mass spectrometry see image 3 . According to the above physicochemical and spectral data, the chemical structure of PAP1 was identified, as shown in Formula I.
[0032] Table 1 PAP1 1 H(500MHz) and 13 C (125MHz) NMR data
[0033]
[0034]
[0035] a) Measured at 500MHz. b) Measured at 125MHz. c) Overlapped signals were reported without designatin...
Embodiment 3
[0036] Example 3 Promoting effect of new polypeptide PAP1 on the proliferation of Balb / c 3T3 cells
[0037] Cells in the logarithmic growth phase (Balb / c 3T3 cell line, purchased from the American Type Culture Collection, ATCC) were digested with trypsin, centrifuged, and resuspended for cell counting, counted as 3000 cells Inoculate each well in a 96-well plate, tap until the cells are evenly dispersed, and block with PBS. 37°C, 5% CO 2 Incubate overnight. Abandon the medium, add starvation medium containing 0.5% FBS by volume to treat for 24h, and synchronize the cells; discard the starvation medium, add complete medium to configure different concentrations of polypeptide PAP1 (prepared in Example 1), 37 ° C, 5 %CO 2 Incubator for 48h. Add CCK8 reagent at 10 μL / well, incubate at 37°C for 3 h, and detect with dual wavelengths of 450 nm and 630 nm on a microplate reader. Calculate the cell survival rate and repeat the experiment at least 3 times;
[0038] Test results su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com