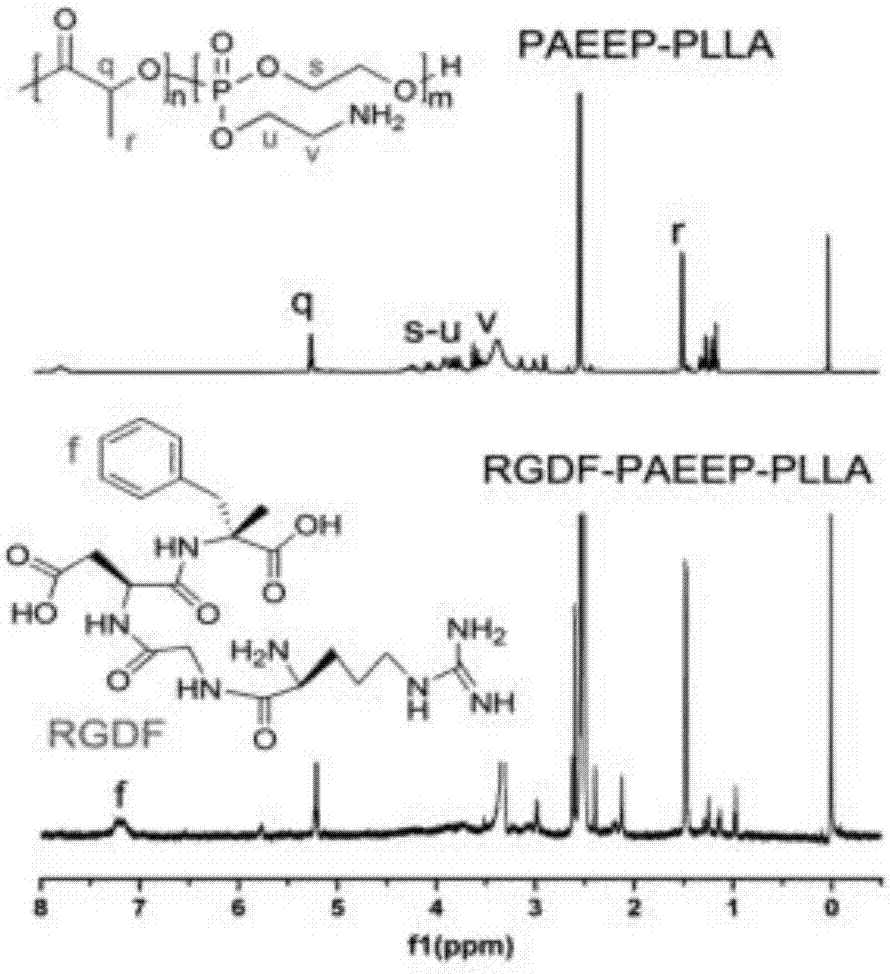
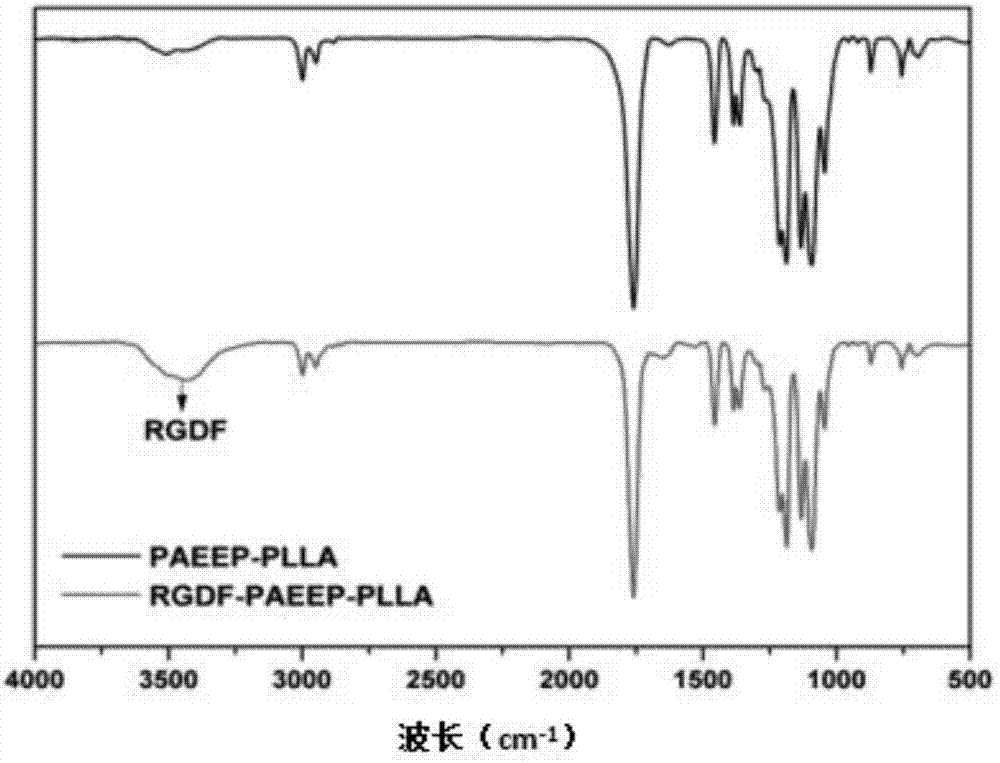
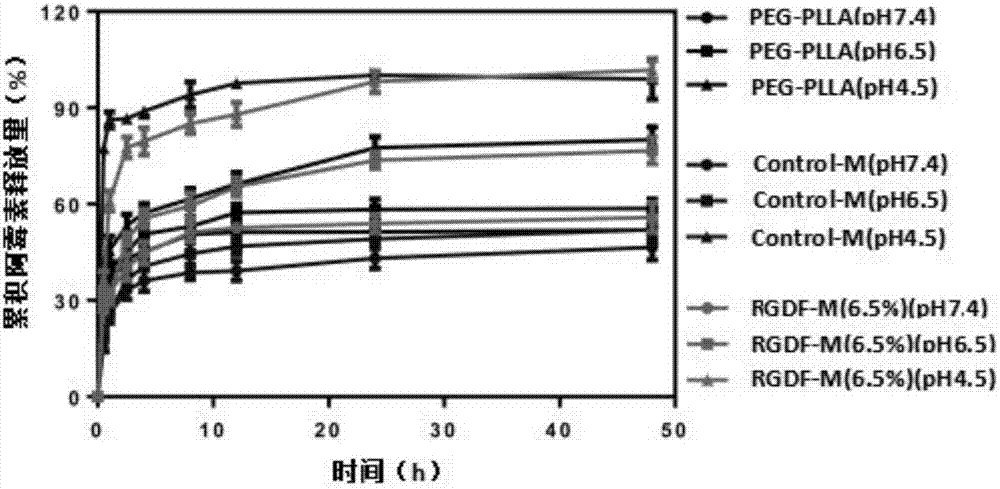
Mixed medicament carrying micelle based on polyphosphoester, preparation method thereof and a mixed medicament carrying micelle modified by positive targeted group
An active targeting group, polyphosphate technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem of PEG lack of active sites, slow drug release, difficult Targeted modification and other issues, to achieve excellent in vivo long-circulation effect, easy targeted modification, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The present invention provides a method for preparing the mixed drug-loaded micelles described in the above scheme, comprising the following steps:
[0087] mixing polyphosphate-polylactic acid diblock copolymers, polyethylene glycol-polylactic acid diblock copolymers, hydrophobic chemotherapeutic drugs, organic basic compounds and organic solvents to obtain an oil phase;
[0088] adding the oil phase to water for emulsification to obtain an emulsion;
[0089] The organic solvent in the emulsion is removed to obtain the mixed drug-loaded micelles based on polyphosphate.
[0090] The present invention combines polyphosphate ester-polylactic acid two-block copolymer (PAEEP-PLLA), polyethylene glycol-polylactic acid two-block copolymer (PEG-PLLA), hydrophobic chemotherapeutic drug, organic basic compound and organic solvent Mix to obtain an oily phase. In the present invention, the mass ratio of the PEG-PLLA and PAEEP-PLLA is preferably (1-20):(1-5), more preferably (2-1...
Embodiment 1
[0112] Mix 1 mol of 2-chloro-2-oxo-1,3,2-dioxaphospholane, 0.9 mol of N-(tert-butoxycarbonyl)ethanolamine and 40 mol of tetrahydrofuran, and carry out substitution reaction at 0°C for 12 hours, After rotary evaporation of the solvent, vacuum drying at 60°C for 20 h under a vacuum of 0.03 atm to obtain 2-(N-(tert-butoxycarbonyl)ethanolamine)-2-oxo-1,3,2-dioxaphospha Cyclopentane (N-Boc-EAOP)
[0113] (2) Mix polylactic acid, N-Boc-EAOP and tetrahydrofuran, and carry out ring-opening polymerization at 60°C for 3 hours under the action of an organoaluminum catalyst to obtain an intermediate product;
[0114] (3) The intermediate product obtained in the step (2) is mixed with tetrahydrofuran and trifluoroacetic acid to carry out a deprotection reaction, and then the material obtained after the deprotection reaction is finished is precipitated with ether, filtered, and the obtained solid Vacuum drying at 40°C for 15 hours under a vacuum degree of 0.03atm to obtain PAEEP 12 -PLLA ...
Embodiment 2
[0118] Weigh respectively doxorubicin hydrochloride 8.2mg, PEG 83 -PLLA 70 (wherein the weight average molecular weight of PEG block is 5000, and the weight average molecular weight of PLLA block is 5000) 60.2mg and the PAEEP that embodiment 1 prepares 12 -PLLA 70 (wherein the weight-average molecular weight of the PAEEP block is 2000, and the weight-average molecular weight of the PLLA block is 5000) 40.2mg, be dissolved in the mixed solvent of 1mL chloroform and 0.5mL methanol together, and add 50 μ L triethylamine solution to dissolve doxorubicin hydrochloride After the element was completely dissolved, it was added dropwise into 15mL ultrapure water and ultrasonic emulsification was performed using a cell disruptor, maintaining a power of 200w for 5min to obtain a uniform and stable emulsion; the emulsion was placed at room temperature and stirred at 150rpm After 24 hours, the organic solvent was evaporated to obtain mixed drug-loaded micelles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com