Oleanolic acid nanometer oral system with inhibiting effect on insulin resistance
A technology for insulin resistance and oleanolic acid, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of large insulin loss and poor oral bioavailability of insulin resistance substances. , easy to be digested, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of embodiment 1 OA-PGA micelle particle
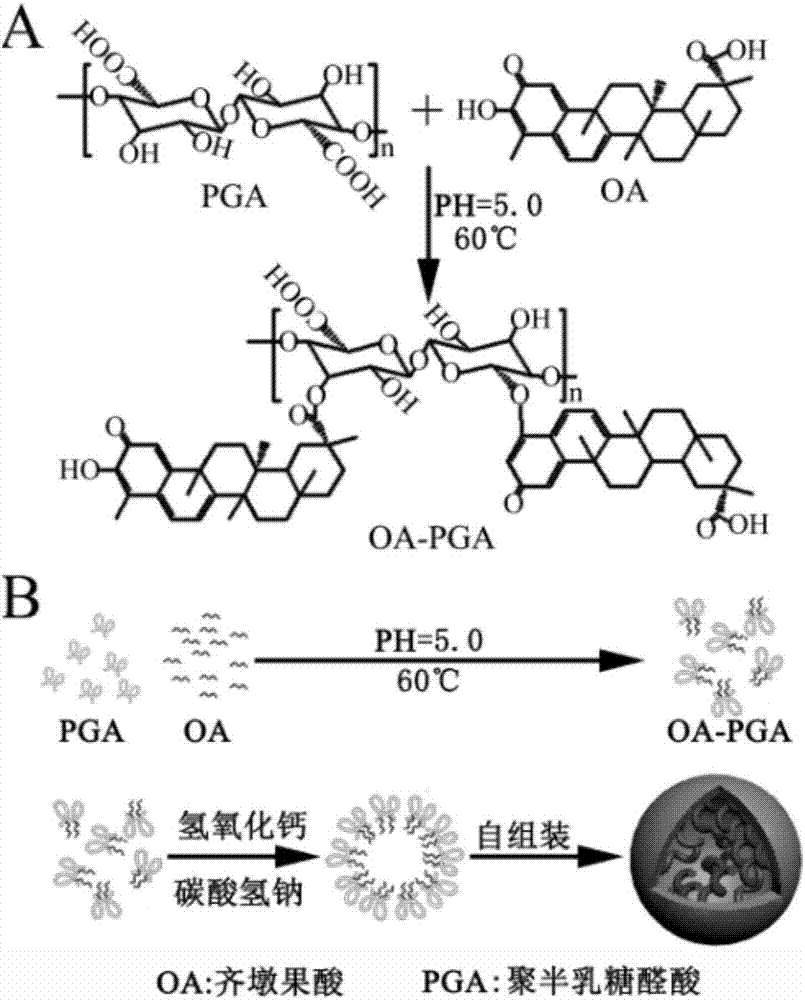
[0072] 1. Schematic diagram of the synthesis of OA-PGA micellar particles figure 1 As shown, the carboxyl group on polygalacturonic acid (PGA) and the hydroxyl group on oleanolic acid (OA) undergo esterification reaction under alkaline conditions to form OA-PGA polymer, because polygalacturonic acid has affinity Oleanolic acid is water-based, and oleanolic acid is hydrophobic. Under the action of different hydrophilic properties at both ends, the OA-PGA polymer self-assembles to form nano-sized micellar particles.
[0073] 2. Specifically, the synthesis method of OA-PGA micellar particles is as follows:
[0074] (1) Dissolve 0.05-0.2g of polygalacturonic acid (pectin can be used instead) in 5mL of double distilled water at 55°C, adjust the pH value to 8.0 with NaOH, and then magnetically stir at 600rpm for 0.5h.
[0075] (2) Dissolve 0.05-0.2 g of oleanolic acid in 5 mL of dimethylformamide (DMF) solvent and st...
Embodiment 2
[0078] The characterization of embodiment 2 OA-PGA micelle particle
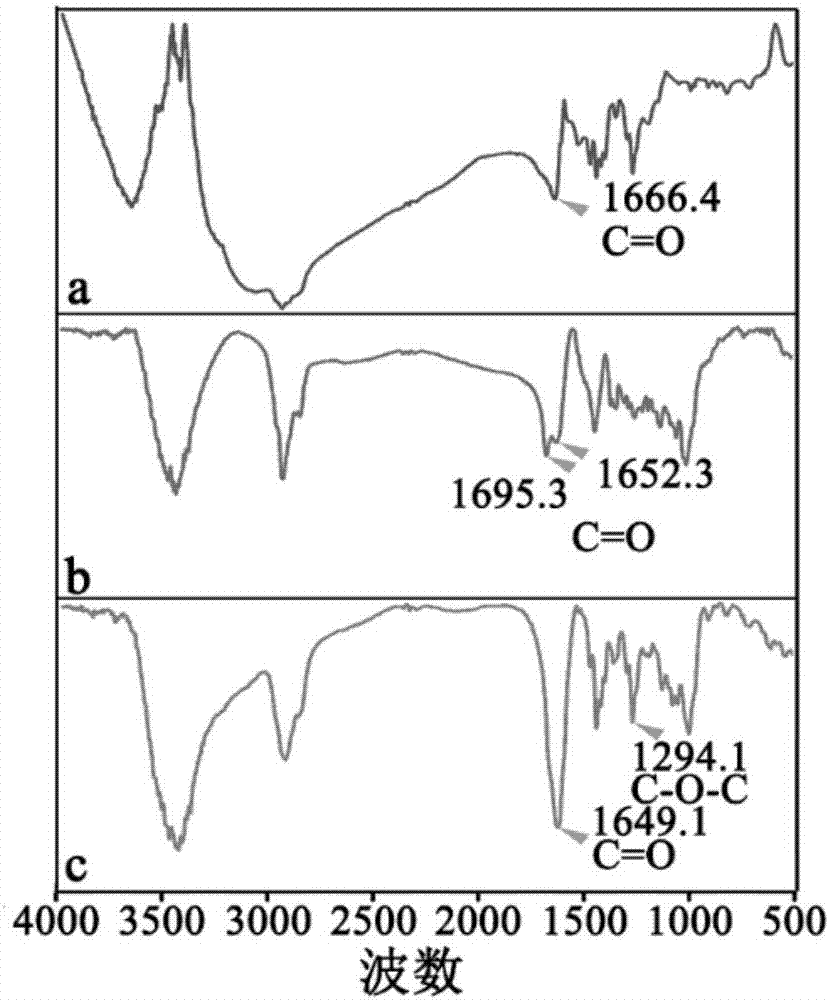
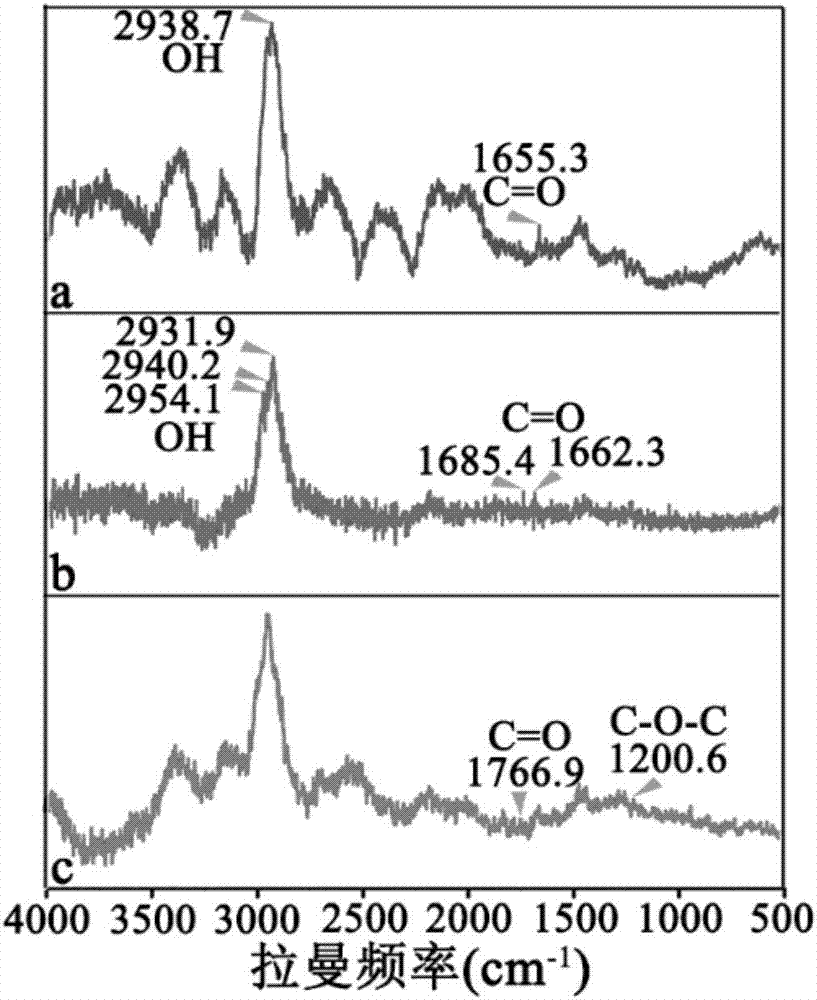
[0079] 1. Infrared spectrum and Raman spectrum detection of OA-PGA micellar particles
[0080] Dry the OA-PGA micellar particles, then put them into a mortar, add a certain amount of KBr, and grind the mixture evenly to make the particle size less than 2 μm, so as to avoid the influence of scattered light, and then put them into a dryer for drying. Press the mixture into a transparent sheet with a pressure of about 10 MPa on the hydraulic press, and measure it on the machine.
[0081] In order to understand the changes in the stage of forming OA-PGA micelles, we performed infrared spectroscopy experiments on OA-PGA micelles, as shown in figure 2As shown, in the process of synthesizing OA-PGA micellar particles, the reaction between substances caused the change of the corresponding characteristic peaks in the infrared spectrum, in OA at 1666.4cm -1 The absorption peak is the C=O stretching fluctuation on t...
Embodiment 3
[0090] Example 3 Experiment of long-term oral delivery of OA-PGA micellar particles in rat model
[0091] 1. Establishment of type 2 diabetes rat model
[0092] Purchasing rats, the incidence rate in the male and female rats of type 2 diabetes according to literature is respectively 92% and 43%, so use STZ (70mg / kg, configure with citric acid buffer) to induce male rats and High-fat feed (feeding for two weeks) was carried out to establish a type 2 diabetes model, and the fasting blood glucose value of rats ≥ 200 mg / dl was considered as a type 2 diabetes rat, which could be used for subsequent experiments.
[0093] 2. Detection of long-term drug feeding signs and oral hypoglycemic experiment in rats
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com