Traditional Chinese medicine preparation for resolving phlegm to relieve cough and preparation method thereof
A technology of traditional Chinese medicine preparations, eliminating phlegm and relieving cough, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. None, the effect of reducing moisture absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] A preparation method of dispersible tablets prepared from a traditional Chinese medicine preparation for eliminating phlegm and relieving cough comprises the following steps:
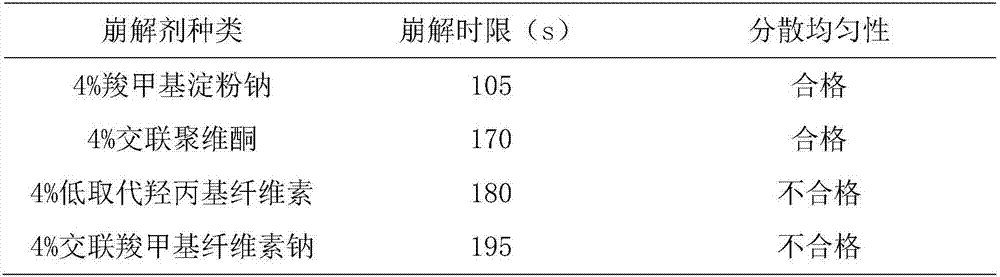
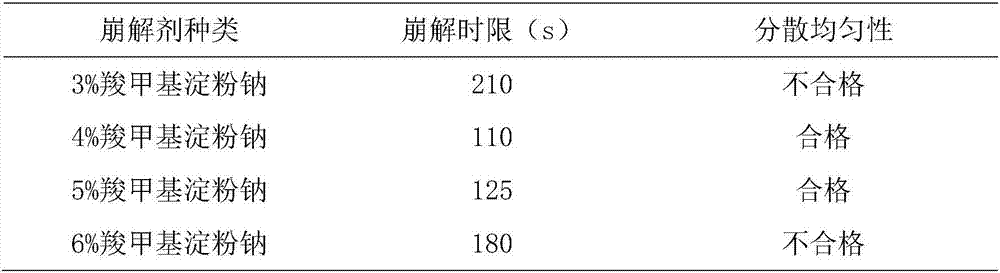
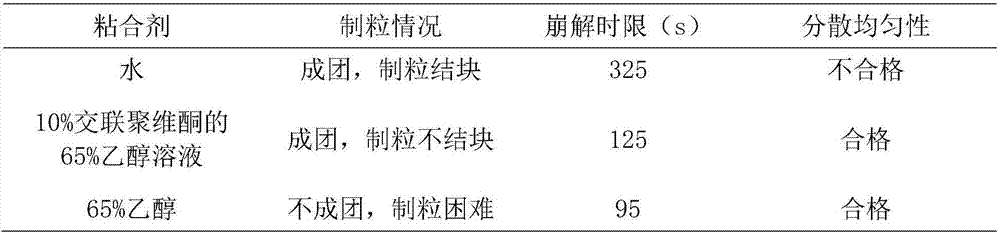
[0071] T1. Take the following components: β-cyclodextrin, crospovidone, hydroxypropyl methyl cellulose, pregelatinized starch, sodium carboxymethyl starch, sodium lauryl sulfate, micronized silica gel, crushed Pass a 100-mesh sieve for use;
[0072] T2. Weigh the components in the following parts by weight: Platycodon grandiflorum 2100g, licorice 1000g, Polygalaceae extract 700g, menthol 5g, orange oil 12g, ephedrine hydrochloride 40g, Baibuliu extract 450g, β-cyclodextrin 120g, Cross-linked povidone 400g, hydroxypropyl methylcellulose 100g, pregelatinized starch 1000g, sodium carboxymethyl starch 150g, sodium lauryl sulfate 120g, micronized silica gel 30g;
[0073] T3. Preparation of dry powder of medicinal extract: take Platycodon grandiflorum and Glycyrrhiza medicinal materials, crush them into 10 m...
Embodiment 2
[0079] A preparation method for preparing dispersible tablets from a traditional Chinese medicine for eliminating phlegm and relieving cough comprises the following steps:
[0080] T1. Take the following components: β-cyclodextrin, crospovidone, hydroxypropyl methyl cellulose, pregelatinized starch, sodium carboxymethyl starch, sodium lauryl sulfate, micronized silica gel, crushed Pass a 100-mesh sieve for use;
[0081] T2. Weigh the components according to the following parts by weight: Platycodon grandiflorum 2400g, licorice 1300g, Polygalaceae extract 900g, menthol 8g, orange oil 14g, ephedrine hydrochloride 60g, Baibuliu extract 650g, β-cyclodextrin 160g, 600g of crospovidone, 200g of hydroxypropyl methylcellulose, 2000g of pregelatinized starch, 250g of sodium carboxymethyl starch, 160g of sodium lauryl sulfate, 50g of micronized silica gel;
[0082] T3. Preparation of dry powder of medicinal extracts: take Platycodon grandiflorum and licorice herbs, crush them into 8 mesh coar...
Embodiment 3
[0088] A preparation method of dispersible tablets prepared from a traditional Chinese medicine preparation for eliminating phlegm and relieving cough comprises the following steps:
[0089] T1. Take the following components: β-cyclodextrin, crospovidone, hydroxypropyl methyl cellulose, pregelatinized starch, sodium carboxymethyl starch, sodium lauryl sulfate, micronized silica gel, crushed Pass a 100-mesh sieve for use;
[0090] T2. Weigh the following components: Platycodon grandiflorum 2250g, licorice 1130g, Polygalaceae extract 800g, menthol 6.7g, orange oil 13g, ephedrine hydrochloride 50g, Baibuliu extract 550ml, β-cyclodextrin 140g , Cross-linked povidone 500g, hydroxypropyl methylcellulose 150g, pregelatinized starch 1500g, sodium carboxymethyl starch 200g, sodium lauryl sulfate 140g, micronized silica gel 40g;
[0091] T3. Preparation of dry powder of medicinal extract: take Platycodon grandiflorum and Glycyrrhiza medicinal materials, crush them into 9 mesh coarse powder, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com