Co-catalyst for preparing hydrogen through photocatalytic decomposition of formic acid, photocatalytic system, and method for preparing hydrogen by decomposing formic acid
A co-catalyst and photocatalytic technology, applied in the field of photocatalysis, to achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Heterojunction CoP x Synthesis of @CN:
[0080] Disperse 500mg CN in 100mL water, add 66mg cobalt chloride hexahydrate (CoCl 2 ·6H 2 (0), stirred for 24 hours, then added 2 mL of the newly prepared borane ammonia complex aqueous solution containing 270 mg borane ammonia complex, stirred at room temperature for about 24 hours until the solution was black, and centrifuged to obtain cobalt nanoparticles loaded on CN The solid (Co@CN) was added, and the obtained Co@CN solid was washed three times with hydrated ethanol, and dried in vacuo. The obtained Co@CN was then fully physically ground with 165 mg of sodium hypophosphite to obtain a solid powder mixture. The powder mixture was placed in a porcelain boat and heated at 300°C for 2 hours under the protection of argon, and the obtained solid was hydrated with water. Washed three times with ethanol and dried in vacuum to obtain heterojunction CoP x @CN, where CoP x The mass fraction of is 5wt%.
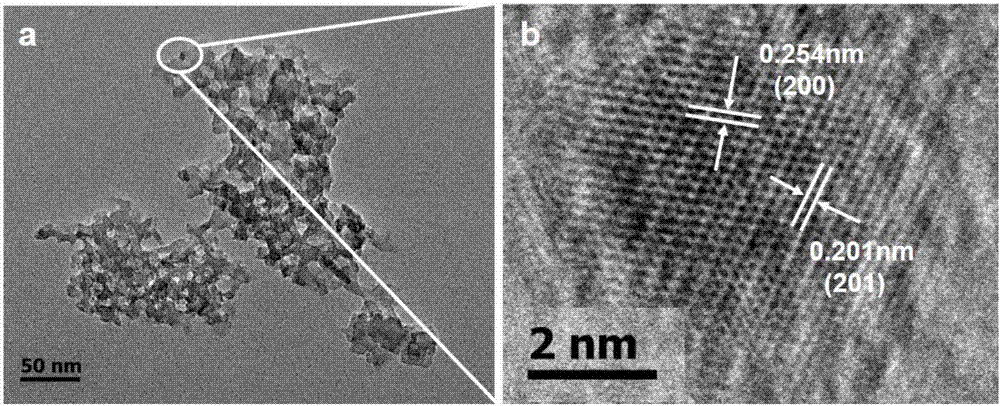
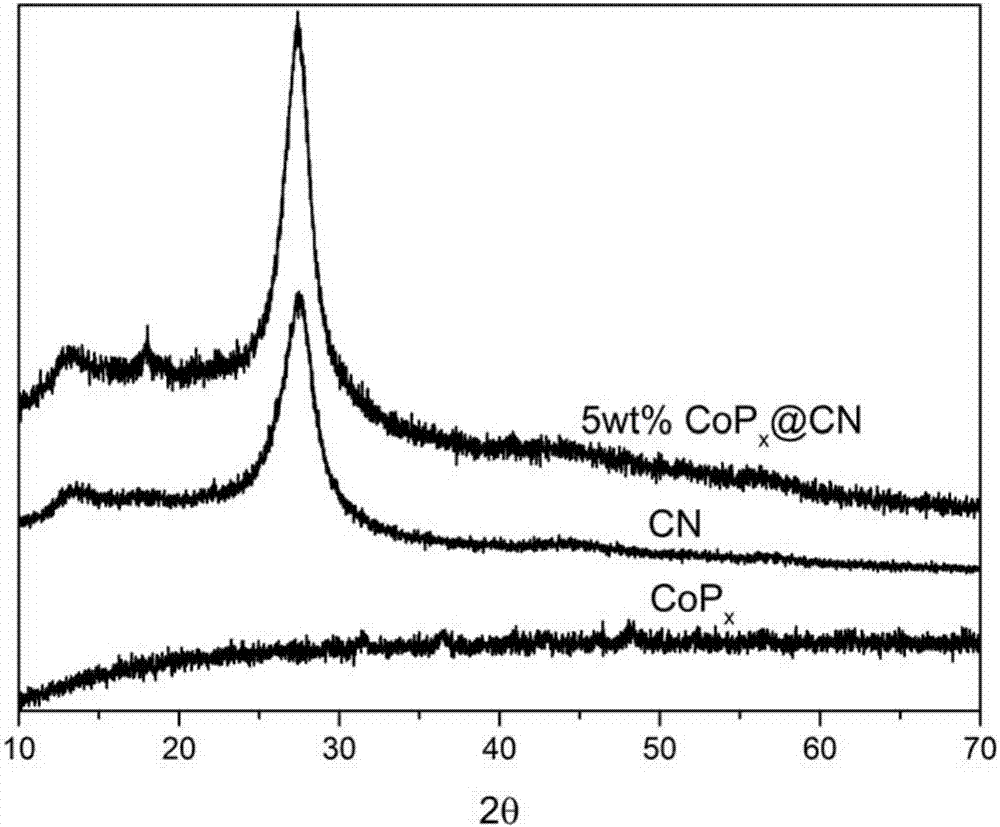
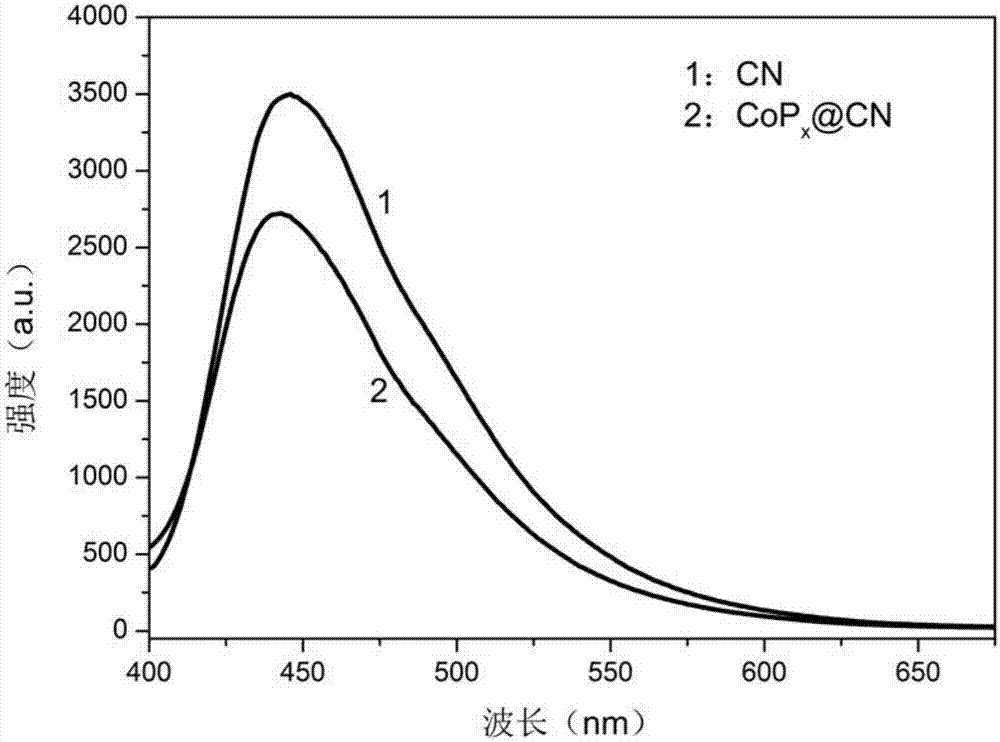
[0081] figure 1 For t...
Embodiment 2
[0085] Heterojunction NiP z Synthesis of @CN - Method 1:
[0086] Using Ni(OH) 2 @CN Synthesis of NiP as a precursor y @CN. 100mgNi(NO 3 )·6H 2 O was dissolved in 100 mL water containing 50 mg sodium citrate, then 200 mg CN was added, stirred overnight, and excess 0.5 M NaOH solution was added dropwise to form a flocculent precipitate, which was centrifuged and dried to obtain Ni(OH) 2 @CN solid. Add 175 mg of sodium hypophosphite to the obtained solid, grind to obtain a mixed powder, put the powder into a porcelain boat, put it in a tube furnace, and heat it at 300°C for 2 hours under the protection of argon, and then wash the obtained solid with deionized water and ethanol were washed three times and dried in vacuum to obtain the heterojunction NiP z @CN.
Embodiment 3
[0088] Heterojunction NiP z Synthesis of @CN - Method 2:
[0089] Synthesis of NiP Using Ni@CN as Precursor y @CN. Disperse 71 mg of CN in 45 mL of water, ultrasonicate for 30 minutes, stir to form a suspension, then add 110 mg of nickel chloride hexahydrate to the suspension, and after stirring for 40 hours, add 5 mL of newly prepared borane ammonium complex containing 450 mg to the solution The borane ammonium complex aqueous solution was stirred at room temperature for 24 hours. The solution was black and centrifuged to obtain a solid (Ni@CN) loaded with nickel nanoparticles on CN. The obtained Co@CN solid was washed three times with hydrated ethanol, vacuum dry. The obtained Ni@CN and 165 mg of sodium hypophosphite were then fully physically ground to obtain a solid powder mixture. The powder mixture was placed in a porcelain boat and heated at 300°C for 2 hours under the protection of argon, and the obtained solid was hydrated with water. Washed three times with ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com