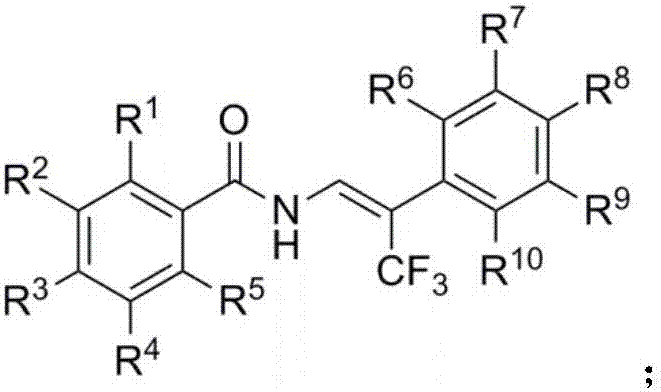
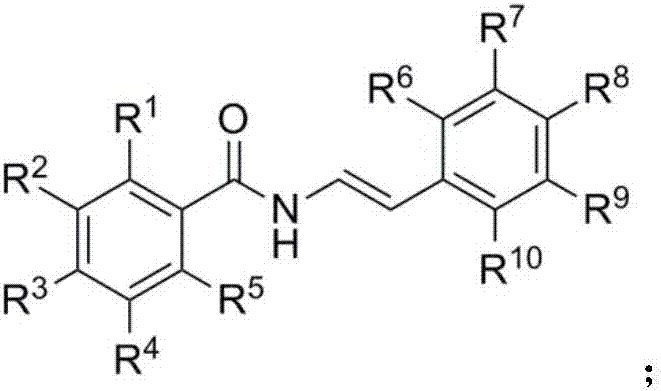
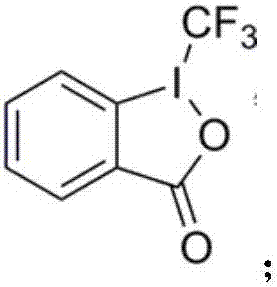
Beta-trifluoromethyl enamine derivative and preparation method thereof
A technology of trifluoromethylenamine and trifluoromethyl, which is applied in the field of β-trifluoromethylenamine derivatives and their preparation, can solve the problems of high technical requirements, difficult to obtain, expensive and the like, and achieves reaction operation And the post-processing process is simple, the reaction conditions are mild, and the various effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of Example 1N-(3,3,3-trifluoro-2-phenyl-1-propenyl)benzamide (2a)
[0045]
[0046] With disodium hydrogen phosphate as base, without adding nitrogen-containing ligand (reaction (1)-(3)) as the control experiment of adding nitrogen-containing ligand (reaction (4)-(6)), specifically as follows:
[0047] (1) Weigh N-(2-phenyl-1-vinyl)benzamide 1a (0.059g, 0.25mmol), Togni reagent (0.119g, 0.375mmol), cuprous chloride (0.003g, 0.025mmol) ) was dissolved in 4 mL of 1,2-dichloroethane (DCE), disodium hydrogen phosphate (0.071 g, 0.5 mmol). The mixture was heated to 90°C, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was separated and purified by silica gel column chromatography (n-hexane:acetone=5:1) to obtain compound 2a. Isolated yield was 52%.
[0048] (2) Weigh N-(2-phenyl-1-vinyl)benzamide 1a (0.059g, 0.25mmol), Togni reagent (0.119g, 0.375mmol), cuprous bromide (0.004g, 0.025mmol)...
Embodiment 2
[0066] Example 2 4-Methyl-N-(3,3,3-trifluoro-2-phenyl-1-propenyl)benzamide (2b)
[0067]
[0068] Weigh 4-methyl-N-(2-phenyl-1-vinyl)benzamide 1b (0.060g, 0.25mmol), Togni reagent (0.119g, 0.375mmol), cuprous iodide (0.005g, 0.025mmol) was dissolved in 4mL 1,2-dichloroethane (DCE), disodium hydrogen phosphate (0.071g, 0.5mmol), DMEDA (0.005g, 0.05mmol). The mixture was heated to 90°C, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was separated and purified by silica gel column chromatography (n-hexane:acetone=5:1) to obtain compound 2b. Isolated yield was 68%.
[0069] 2b: 68% yield; 1 H NMR (400MHz, CDCl 3 )δ: 7.94(d, J=10.4Hz,1H),7.77(d,J=10.1Hz,1H),7.55–7.46(m,5H),7.40(d,J=6.9Hz,2H),7.22( d,J=8.0Hz,2H),2.38(s,3H); 13 CNMR (101MHz, CDCl 3 )δ: 164.24(s), 143.65(s), 130.10(s), 129.62(s), 129.59(s), 129.46(s), 129.37(s), 128.89(s), 127.20(s), 126.73( q, J=7.07Hz), 124.19(q, J=270.7Hz), 112.51(q...
Embodiment 3
[0070] Example 3 Synthesis of 4-methoxy-N-(3,3,3-trifluoro-2-phenyl-1-propenyl)benzamide (2c)
[0071]
[0072] Weigh 4-methoxy-N-(2-phenyl-1-vinyl)benzamide 1c (0.063g, 0.25mmol), Togni reagent (0.119g, 0.375mmol), cuprous iodide (0.005g , 0.025mmol) was dissolved in 4mL 1,2-dichloroethane (DCE), disodium hydrogen phosphate (0.071g, 0.5mmol), DMEDA (0.005g, 0.05mmol). The mixture was heated to 90°C, and the reaction was followed by TLC until the reaction was completely completed. After the reaction, the crude product was separated and purified by silica gel column chromatography (n-hexane:acetone=5:1) to obtain compound 2c. Isolated yield was 61%.
[0073] 2c: 61% yield; 1 H NMR (400MHz, CDCl 3 )δ: 7.96–7.83(m,1H),7.74(d,J=11.3Hz,1H),7.56(t,J=7.9Hz,2H),7.53–7.43(m,3H),7.40(d,J =7.1Hz, 2H), 6.89(d, J=8.7Hz, 2H), 3.82(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 163.68(s), 163.23(s), 130.18(s), 129.62(s), 129.33(s), 129.19(s), 126.83(q, J=7.0Hz), 124.39(s), 124.21( q, J=270....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com