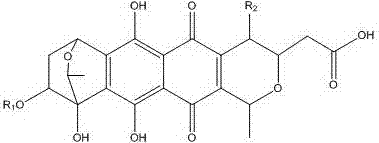
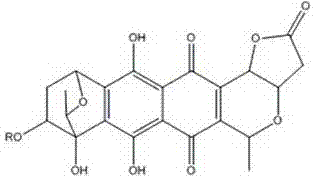
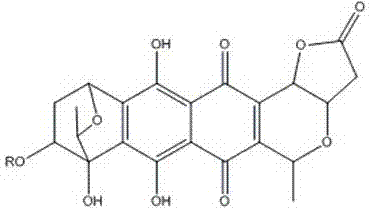
Medical application of granaticin type compound
A technology for compounds and uses, applied in the field of medical use of punicillin compounds, can solve the problems of different structures, low sequence similarity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]Example 1 Extraction and preparation of durianin compounds A-F of the present invention
[0061] Preparation of seed solution of N01W-352
[0062] The slant culture or spore liquid of strain N01W-352 was inoculated on the seed medium, and cultured at 28 °C and 200 rpm for 48 h to obtain the seed liquid;
[0063] The seed medium mentioned therein was prepared as follows: 20.0 grams of corn starch, 4.0 grams of glucose, 3.0 grams of peptone, 4.0 grams of beef extract, 2.0 grams of yeast extract powder, added water to 1000 mL; pH value 7.0, 121 ℃ Sterilize for 30 minutes;
[0064] Preparation of fermented broth of N01W-352
[0065] The above seed liquid was inoculated into the fermentation medium with an inoculation amount of 8% by volume, and fermented in a shake flask or a fermenter at a fermentation temperature of 27°C and a fermentation time of 120 h;
[0066] The fermentation medium mentioned therein was prepared according to the following method: 30.0 grams of gluc...
Embodiment 2
[0084] Example 2 Determination of the inhibitory activity of the duracin compounds of the present invention on IDO or TDO
[0085] Using genetic engineering technology, construct expression plasmids containing human IDO and human TDO genes, carry out recombinant expression in Escherichia coli, isolate and purify recombinant human IDO and TDO, and establish an activity detection system for IDO or TDO. Activity method of IDO or TDO Detection method: 96-well plate method is adopted, and the detection buffer contains 50 mM potassium phosphate buffer (pH 6.5) containing 40 mM vitamin C, 400 μg / ml hydrogen peroxide and 20 μM methylene blue. Add an appropriate amount of IDO or TDO to the detection buffer, make an enzyme mixed solution and add it to a 96-well plate, then add the substrate L-tryptophan, react at 37°C for 60min, add 30% (w / v) Trichloroacetic acid terminated the reaction. Incubate the 96-well plate at 65°C for 15 minutes to complete the conversion from formylkynurenine ...
Embodiment 3
[0088] Example 3 Inhibitory activity of durugin compounds of the present invention on IDO induced by gamma-interferon in Hela cells
[0089] Hela cells were cultured in a 96-well plate, the medium was high-sugar DMEM containing 50 U / ml penicillin, 50 U / ml streptomycin, 10% FBS, and the culture conditions were 37°C, 5% CO 2 . Stimulate Hela cells with γ-interferon at a concentration of 200IU, use IDO inhibitor 1-methyltryptophan (l-MT) as a positive drug, add different concentrations of positive drugs and compounds and 50 μM substrate L-tryptophan After culturing for 48 hours, remove the cell culture supernatant, add 30% (w / v) trichloroacetic acid, and incubate the reaction system at 65°C for 15 minutes to complete the transformation from formylkynurenine to kynurenine For conversion, centrifuge at 12,000rpm for 10min, take the supernatant and mix with an equal volume of 2% (w / v) acetic acid solution of p-dimethylaminobenzaldehyde to react, detect the OD value at 490nm, and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com