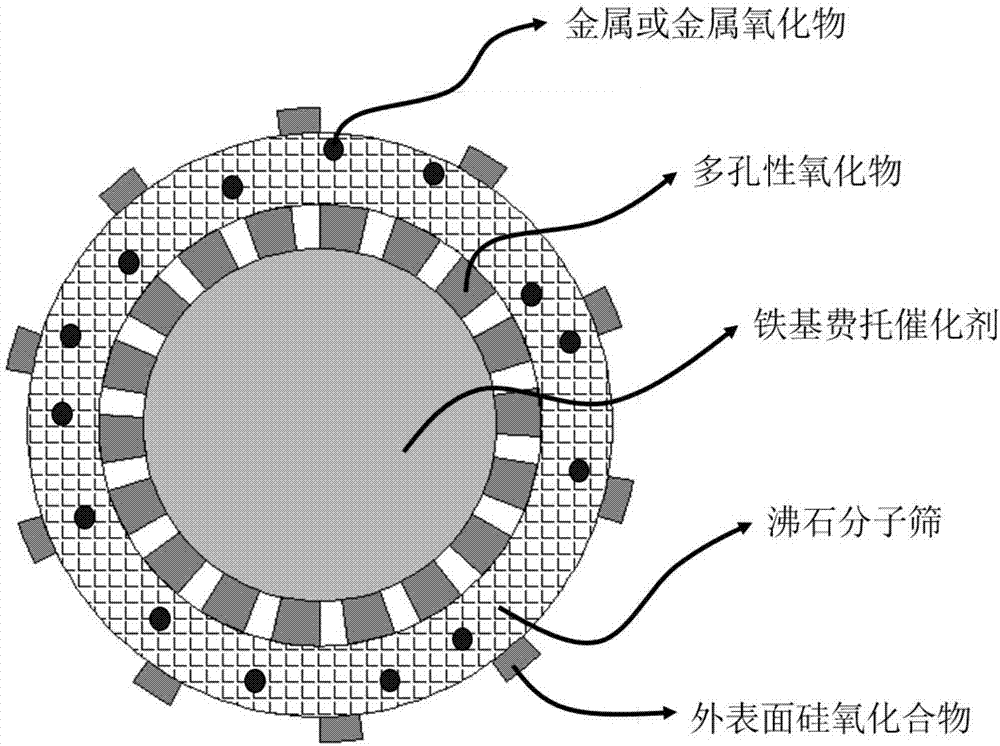
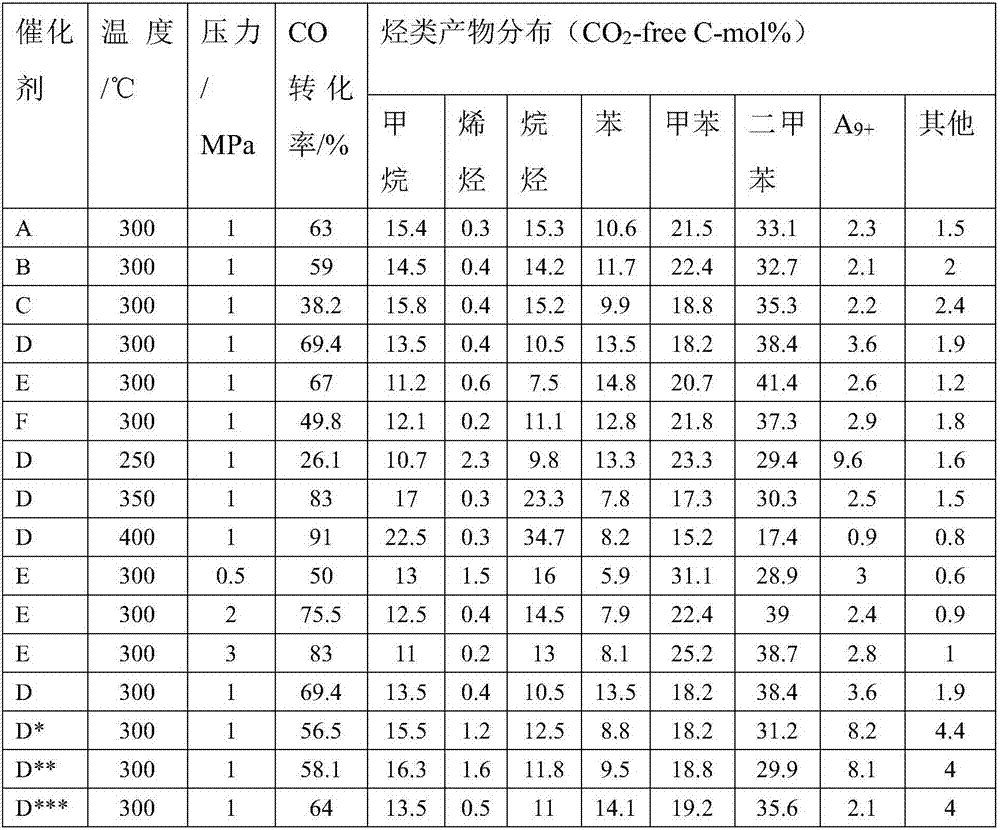
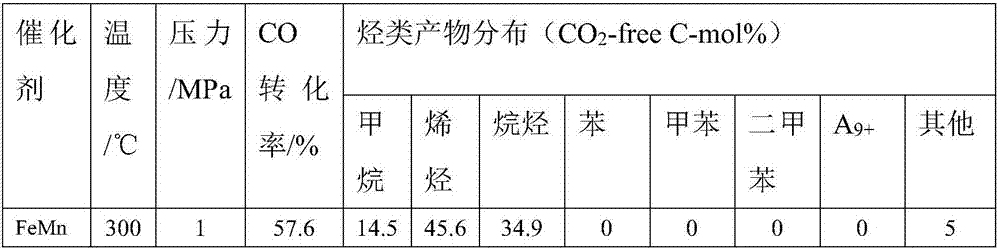
Multistage nano-reactor catalyst for direct preparation of aromatic compounds from synthetic gas, and preparation method and application thereof
A technology of aromatic compounds and nano-reactors, applied in the direction of carbon compound catalysts, catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, etc., can solve intermediate product escape, non-activation reaction, and final aromatics selection high aromatics selectivity and low methane selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve a certain amount of ferric nitrate and manganese nitrate in deionized water, use ammonia water as a precipitant to precipitate at pH = 8.0, age, filter, wash and dry at 120°C for 12 hours, and finally bake at 500°C for 5 hours A precipitated FeMn catalyst with an atomic ratio of iron to manganese of 96 to 4 was obtained. The prepared catalyst is impregnated in a certain amount of tetraethyl orthosilicate solution, and stirred continuously, and then the solvent is removed by rotary evaporation and dried and roasted to obtain SiO 2 Coated samples. Then the sample was impregnated in a template containing tetrapropylammonium hydroxide, tetraethyl orthosilicate, Al 2 o 3 , NaOH, H 2 In the solution of O, among which tetrapropyl ammonium hydroxide, tetraethyl orthosilicate, Al 2 o 3 , NaOH, H 2 The ratio of O is 0.3:1.0:0.03:0.015:130, stirred for 4 hours, then put into a hydrothermal kettle, sealed, heated to 180°C, and hydrothermally crystallized for 48 hours...
Embodiment 2
[0027] Get the precipitation type FeMn catalyst that makes in the last example, place in a certain amount of aluminum isopropoxide trihydrate and keep stirring, then rotary evaporation removes solvent and dry roasting, obtain the 2 o 3Coated samples. Then place the sample in a template containing tetrapropylammonium hydroxide template, tetraethyl orthosilicate, Al 2 o 3 , NaOH, H 2 In the solution of O, among which tetrapropyl ammonium hydroxide, tetraethyl orthosilicate, Al 2 o 3 , NaOH, H 2 The ratio of O is 0.3:1.0:0.03:0.015:130, stirred for 4 hours, then put into a hydrothermal kettle, sealed, heated to 180°C, and hydrothermally crystallized for 48 hours. After the crystallization was completed and cooled, the solid product was filtered, washed until the pH of the washing solution was 8, then dried at 120°C for 12 hours, and then calcined at 500°C for 5 hours to obtain a core layer with a weight fraction of 64% and a transition layer with a weight fraction of 8%. C...
Embodiment 3
[0029] Preparation of 20wt%Fe1wt%K / SiO by Equal Volume Impregnation Method 2 Supported iron-based catalysts. Finally, the sample is placed in a certain amount of aluminum isopropoxide trihydrate and stirred continuously, and then the solvent is removed by rotary evaporation and dried and roasted to obtain a 2 o 3 Coated samples. Then place the sample in a template containing tetrapropylammonium hydroxide template, tetraethyl orthosilicate, Al 2 o 3 , NaOH, H 2 In the solution of O, among which tetrapropyl ammonium hydroxide, tetraethyl orthosilicate, Al 2 o 3 , NaOH, H 2 The ratio of O is 0.3:1.0:0.05:0.010:130, stirred for 4 hours, then put into a hydrothermal kettle, sealed, heated to 180°C, and hydrothermally crystallized for 48 hours. After the crystallization was completed and cooled, the solid product was filtered, washed until the pH of the washing solution was 8, then dried at 120°C for 12 hours, and then calcined at 500°C for 5 hours to obtain a core layer wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com