Triphenylamine derivative, preparation method and application thereof, and preparation method of triphenylamine derivative doped film
A technology of triphenylamine and derivatives, which is applied in the field of preparation of triphenylamine derivatives and their doped thin films, can solve the problems of small color difference, low fluorescence quenching rate, and low optical contrast, and achieve large color difference and fluorescence quenching rate. High and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
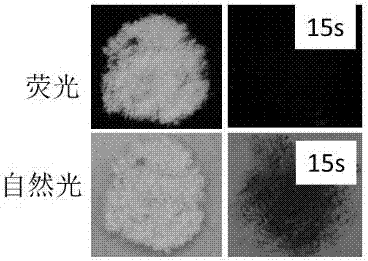
[0035] Dissolve the solid powder of triphenylamine derivative (I) in tetrahydrofuran solvent to prepare a concentration of 1×10 -4 mol / L. Then take 0.01 mL and add it to a 10 mL volumetric flask, then add HCl aqueous solution (9.99 m) L with a pH value of 0.98, and test after 3 minutes, the fluorescence of the solution is completely quenched (the quenching rate is greater than 97%). (For details, please see figure 1 a)
Embodiment 2
[0037] Dissolve 0.1 g of solid powder of triphenylamine derivative (I) in 3 mL of tetrahydrofuran solvent to prepare a concentration of 1×10 - 5 mol / L. Then take 0.01 mL and add it to a 10 mL volumetric flask, then add HCl aqueous solution (9.99 m) L with a pH value of 3.34, and test after 3 minutes, the fluorescence intensity of the solution is significantly reduced (the quenching rate is greater than 50%). (For details, please see figure 1 b)
Embodiment 3
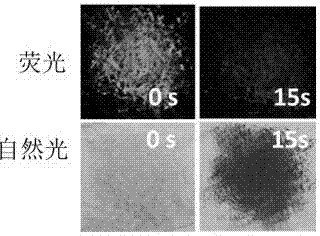
[0039] Spread 0.05 g of the triphenylamine derivative solid powder (I) of the present invention on a quartz plate, it is light yellow under natural light, and shows bright green fluorescence under ultraviolet light. After being placed in 10 ppm hydrogen chloride atmosphere for 15 s, its fluorescence became dark red with a quenching rate of 83.4%. The powder also turns deep red under natural light, and the ∆E reaches 18.7. (see attached figure 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com