Preparation process of amlodipine besylate tablet
A technology of amlodipine besylate tablets and amlodipine besylate, applied in pill delivery, cardiovascular system diseases, drug combination, etc., can solve the problems of poor therapeutic effect of variant angina pectoris, achieve excellent therapeutic effect, Guarantee the effect of product quality and reasonable design of preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
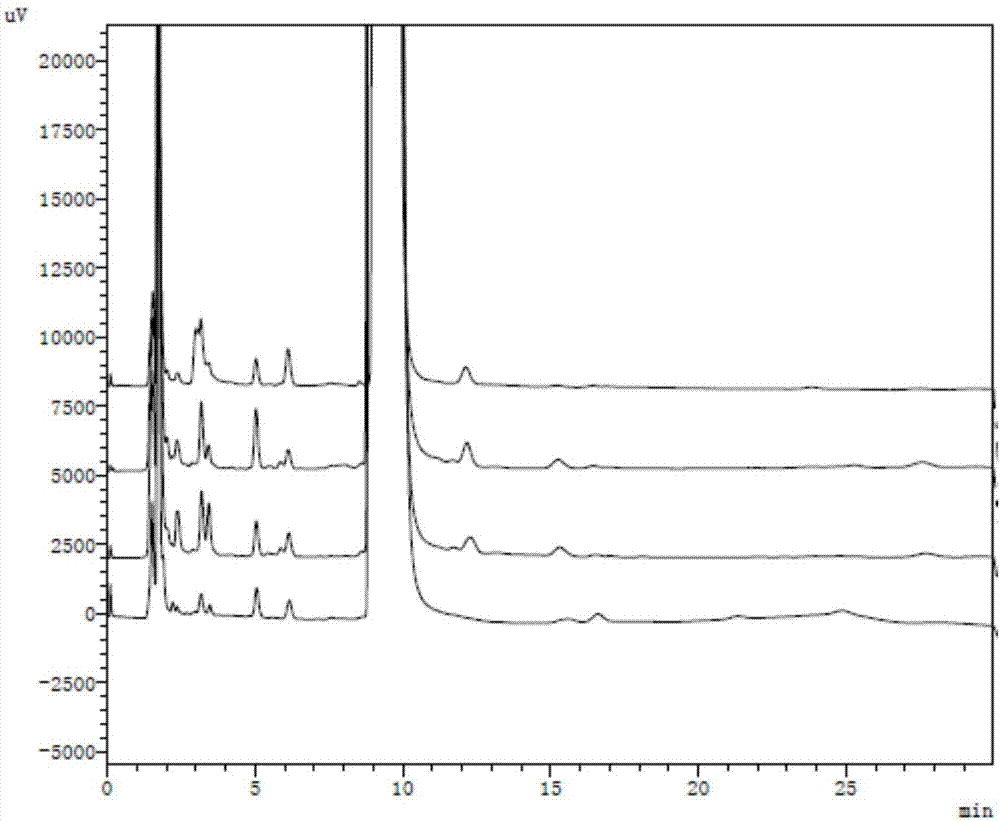
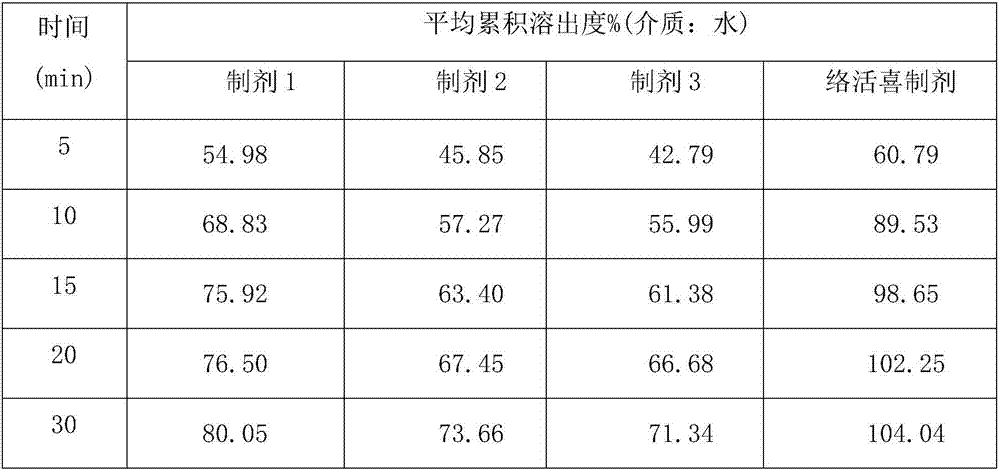
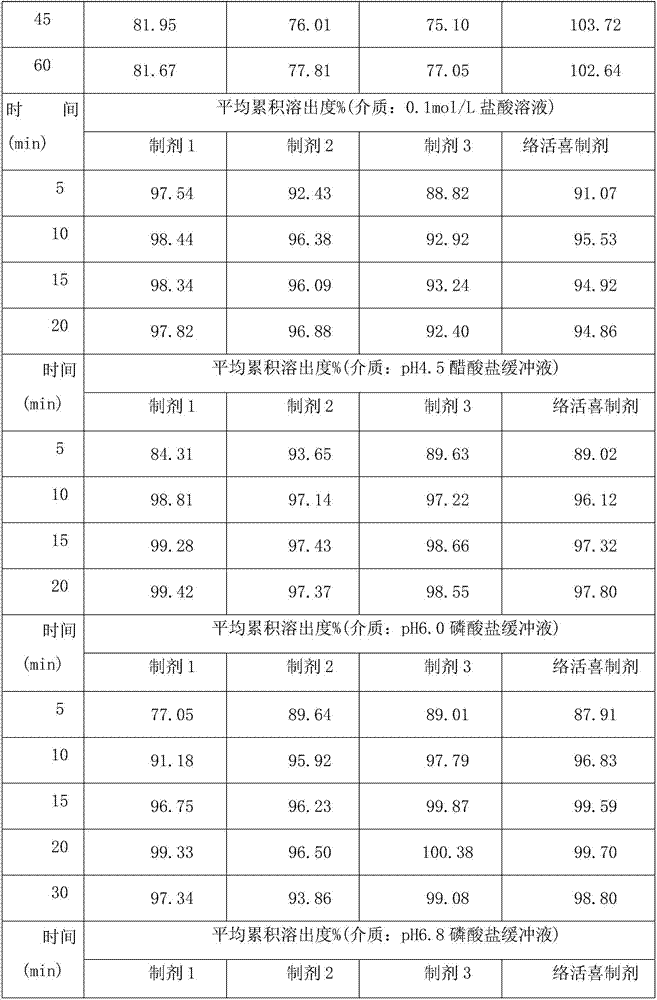
Image
Examples
Embodiment 1
[0028] A kind of preparation technology of amlodipine besylate sheet, comprises the following steps:
[0029] (1) Material preparation process: the amlodipine besylate sheet is made of the following raw materials: amlodipine besylate, microcrystalline cellulose, starch, anhydrous calcium hydrogen phosphate, sodium carboxymethyl starch, starch paste, Medicinal alcohol, magnesium stearate and talc;
[0030] The workshop issues batch production instructions and batch packaging instructions according to the production plan of the production department and fills in the requisition list respectively. The preparation workshop executes the requisition list and goes to the raw and auxiliary material warehouse to receive amlodipine besylate, microcrystalline cellulose, starch, and anhydrous hydrogen phosphate Calcium, carboxymethyl starch sodium, starch paste, medicinal ethanol, magnesium stearate and talcum powder; get PVC hard sheets, aluminum foil, instructions, small boxes, and cart...
Embodiment 2
[0076] A kind of preparation technology of amlodipine besylate sheet, what embodiment 2 is different from embodiment 1 is: step (3) weighing batching process: take by weighing following raw material according to parts by weight: amlodipine besylate 3kg, Starch 30kg, anhydrous calcium hydrogen phosphate 6kg, carboxymethyl starch sodium 8kg, microcrystalline cellulose 25kg, starch paste 4kg, medicinal ethanol 10kg, magnesium stearate 0.1kg, talcum powder 0.1kg; step (4) wet granulation Process: including the following steps: take 3kg of starch and put it into a granulator, add 3kg of amlodipine besylate, 6kg of anhydrous calcium hydrogen phosphate, mix for 5 minutes, then add 4kg of sodium carboxymethyl starch and mix for 5 minutes, then add microcrystalline 10kg of cellulose, mixed for 5 minutes, finally added the remaining starch and microcrystalline cellulose and mixed for 30 minutes, added 50kg of binder, started the stirring level I, chopped the first level, mixed for 600 se...
Embodiment 3
[0078] A kind of preparation technology of amlodipine besylate sheet, what embodiment 3 is different from embodiment 1 is: step (3) weighing batching process: take by weight following raw material: amlodipine besylate 4kg, 40kg of starch, 3.5kg of anhydrous calcium hydrogen phosphate, 15kg of sodium starch glycolate, 32kg of microcrystalline cellulose, 8kg of starch paste, 15kg of medicinal ethanol, 1kg of magnesium stearate, 1kg of talcum powder; step (4) wet granulation process : comprising the following steps: get 5kg of starch and put it into a granulator, add 4kg of amlodipine besylate, 3.5kg of anhydrous calcium hydrogen phosphate, mix for 5 minutes, then add 6kg of sodium starch glycolate and mix for 5 minutes, then add microcrystalline 15kg of cellulose, mixed for 5 minutes, and finally added the remaining starch and microcrystalline cellulose and mixed for 30 minutes, added 50kg of binder, started the stirring stage I, chopped the stage I, mixed for 600 seconds, and ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com