Method for preparing BTX and co-producing tetramethylbenzene from C9<+> heavy aromatic hydrocarbons
A technology of mesitylene and heavy aromatics, applied in the field of aromatics chemical production, can solve the problems of high added value of products, low conversion rate of raw materials, large output of dry gas, etc., and achieve high yield of target products, high conversion rate of raw materials, dry gas The effect of low gas yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
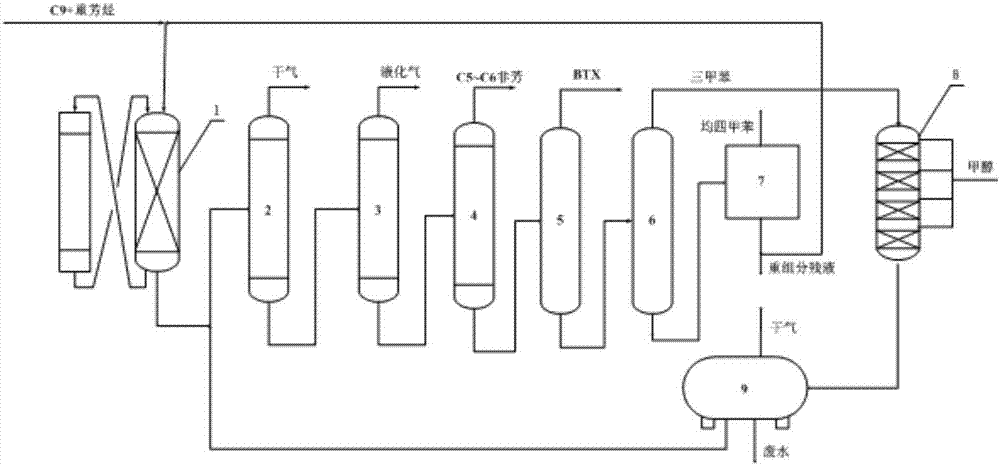
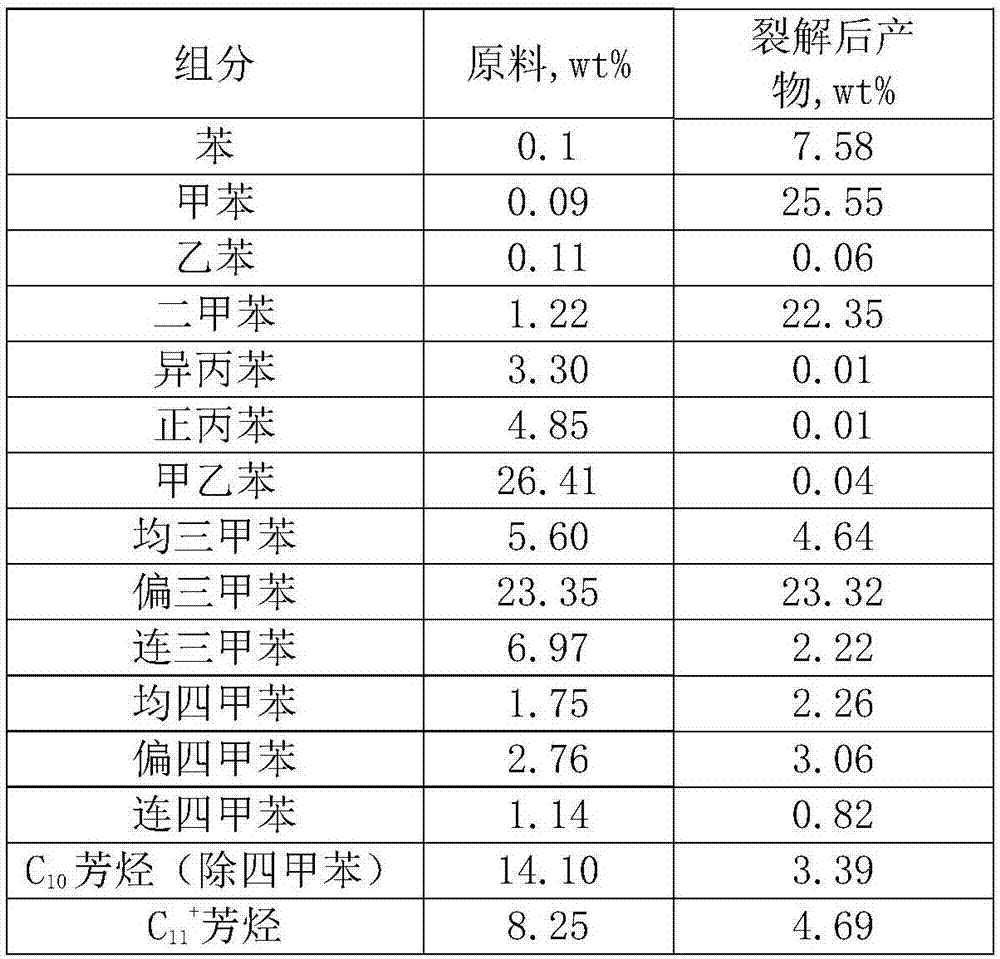
[0030] Take C from a refinery 9 + Heavy aromatics as raw material, load 2% La after 500℃ hydrothermal treatment 2 O 3 Nano ZSM-5 molecular sieve (SiO 2 / Al 2 O 3 The molar ratio is 30) as the catalyst, the reaction temperature is 460℃, the pressure is 0.5MPa, and the raw material mass space velocity is 1.0h -1 Under the conditions of the catalytic cracking reaction, C 9 + The properties of the raw materials and the composition of the liquid phase products after cracking are shown in Table 1.
Embodiment 2
[0032] Catalytic cracking catalyst adopts 450℃ hydrothermal treatment to load 3% La 2 O 3 Nano ZSM-5 molecular sieve (SiO 2 / Al 2 O 3 The molar ratio is 30) catalyst, the reaction conditions are: temperature 450℃, pressure 0.2MPa, raw material mass space velocity 0.5h -1 .
[0033] Alkylation reactor is filled with 2% P 2 O 5 Modified ZSM-5 / MCM-22 composite molecular sieve (the mass ratio of ZSM-5 molecular sieve to MCM-22 molecular sieve is 2:1) catalyst, the reaction conditions are: reaction temperature 360℃, pressure 0.1MPa, raw material mass space velocity 1.0h -1 , The molar ratio of trimethylbenzene to methanol is 1:1.
[0034] After the above catalytic cracking, alkylation and separation processes, the yield of each product is shown in Table 2.
Embodiment 3
[0036] Catalytic cracking catalyst adopts 500℃ hydrothermal treatment to load 2% La 2 O 3 Nano ZSM-5 molecular sieve (SiO 2 / Al 2 O 3 The molar ratio is 30) the catalyst, the reaction conditions are: temperature 500℃, pressure 0.3MPa, raw material mass space velocity 1.0h -1 .
[0037] Alkylation reactor is filled with 3% P 2 O 5 Modified ZSM-5 / MCM-22 composite molecular sieve (the mass ratio of ZSM-5 molecular sieve to MCM-22 molecular sieve is 2:1) catalyst, the reaction conditions are: reaction temperature 420℃, pressure 0.2MPa, raw material mass space velocity 1.5h -1 , The molar ratio of trimethylbenzene to methanol is 2:1.
[0038] After the above catalytic cracking, alkylation and separation processes, the yield of each product is shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com