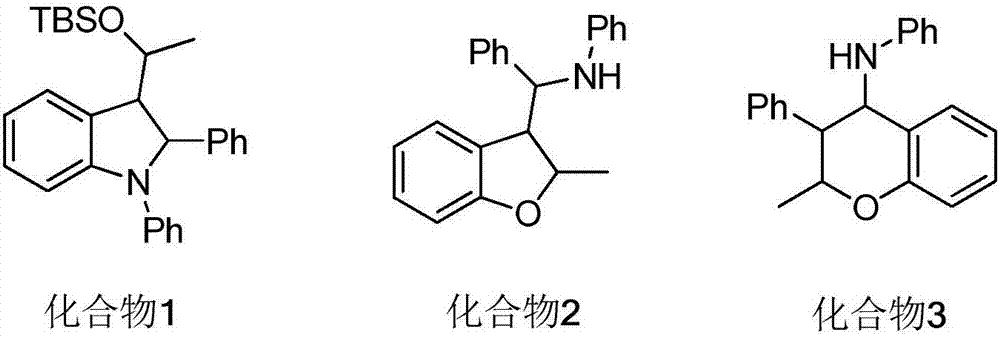
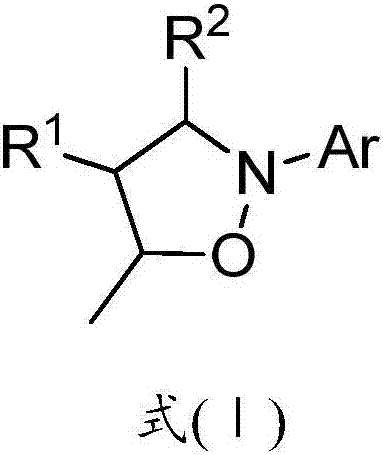
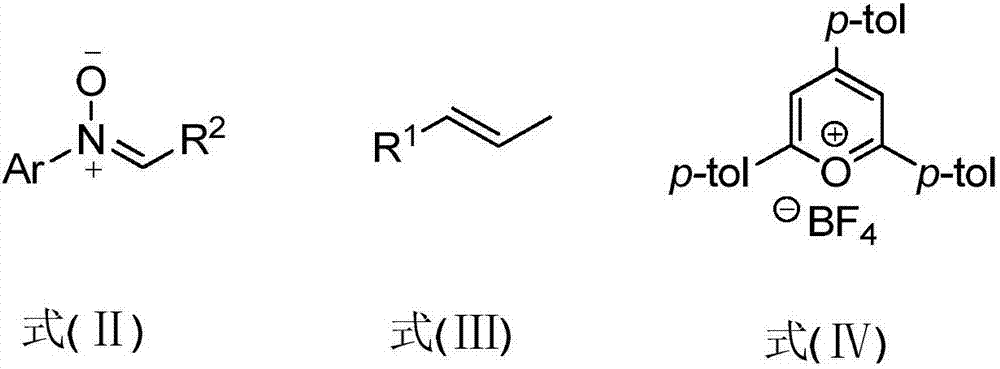
Polysubstituted isoxazolidine derivative and preparation method thereof
A technology for isoxazolidine and derivatives, which is applied in the field of polysubstituted isoxazolidine derivatives and their preparation, can solve the problems of large equipment requirements, harsh conditions and high energy consumption, and achieves simple method, mild conditions and high yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Into a 7mL round bottom flask, add 59mg (0.3mmol) diphenylnitrone, 53mg (0.45mmol) β-methylstyrene, 1.2mg (0.0075mmol) photocatalyst and 4mL dichloromethane, stir well and pass nitrogen After 30 minutes, it was placed under blue LEDs light, and reacted with light at room temperature for 12 hours. After the reaction was completed, the reaction solvent was concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether: ethyl acetate with a volume ratio of 50:1 was used as an eluent to carry out purification and separation by silica gel column chromatography to obtain the corresponding poly Substituted isoxazolidine derivatives, whose reaction formula is:
[0031]
[0032] The product had a purity of 99% and a yield of 85%. NMR data analysis is: 1 H NMR (400MHz, CDCl 3 ):δ H 7.31-7.25 (m, 2H), 7.18-7.16 (m, 5H), 7.11-7.06 (m, 5H), 6.97 (t, J = 7.3Hz, 1H), 6.83-6.82 (m, 2H), 4.94 ( d, J=8.8Hz, 1H), 4.67(dq, J=9.4, 6.0Hz, 1H), 3.63(t...
Embodiment 2
[0035] Into a 7mL round bottom flask, add 59mg (0.3mmol) diphenylnitrone, 47mg (0.45mmol) styrene, 1.2mg (0.0075mmol) photocatalyst and 4mL dichloromethane, stir well and pass nitrogen gas for 30 minutes and place Under the light of blue LEDs, light reaction was carried out at room temperature for 17 h. After the reaction was completed, the reaction solvent was concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether: ethyl acetate with a volume ratio of 30:1 was used as an eluent for purification and separation by silica gel column chromatography to obtain the corresponding poly Substituted isoxazolidine derivatives, whose reaction formula is:
[0036]
[0037] The product had a purity of 99% and a yield of 52%. The NMR data analysis is as follows: 1 H NMR (400MHz, CDCl 3 ):δ H 7.29-7.24(m,3H),7.16-7.10(m,5H),6.95-6.92(m,2H),4.99(d,J=7.9Hz,1H),4.50(t,J=7.6Hz,1H) ,4.33-4.29(m,1H),4.07(dd,J=14.2, 7.1Hz,1H).
[0038] 13 C NMR (10...
Embodiment 3
[0040] Into a 7mL round bottom flask, add 59mg (0.3mmol) diphenylnitrone, 53mg (0.45mmol) indene, 1.2mg (0.0075mmol) photocatalyst and 6mL dichloromethane, stir well, pass nitrogen gas for 30 minutes and place Under the light of blue LEDs, light reaction was carried out at room temperature for 12 hours. After the reaction was completed, the reaction solvent was concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether: ethyl acetate with a volume ratio of 40:1 was used as an eluent for purification and separation by silica gel column chromatography to obtain the corresponding poly Substituted isoxazolidine derivatives, whose reaction formula is:
[0041]
[0042] The product had a purity of 99% and a yield of 81%. The NMR data analysis is as follows: 1 H NMR (400MHz, CDCl 3 ):δ H 7.23-7.16(m,9H),7.06(t,J=8.2Hz,3H),6.93(t,J=7.3Hz,1H), 6.76(t,J=7.5Hz,1H),6.03(d,J =7.7Hz,1H),5.22(td,J=6.4,2.1Hz,1H), 5.01(d,J=8.7Hz,1H),4.44-4.40(m,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com