Aldosterone derivatives, immunogens, and synthesis methods, specific antibodies, detection reagents, preparation methods, and kits
An aldosterone and derivative technology, applied in biological testing, immunoglobulins, chemical instruments and methods, etc., can solve the problems of unsuitability for large-scale clinical sample determination, high testing cost, poor stability, etc., to meet the needs of clinical testing, High sensitivity and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
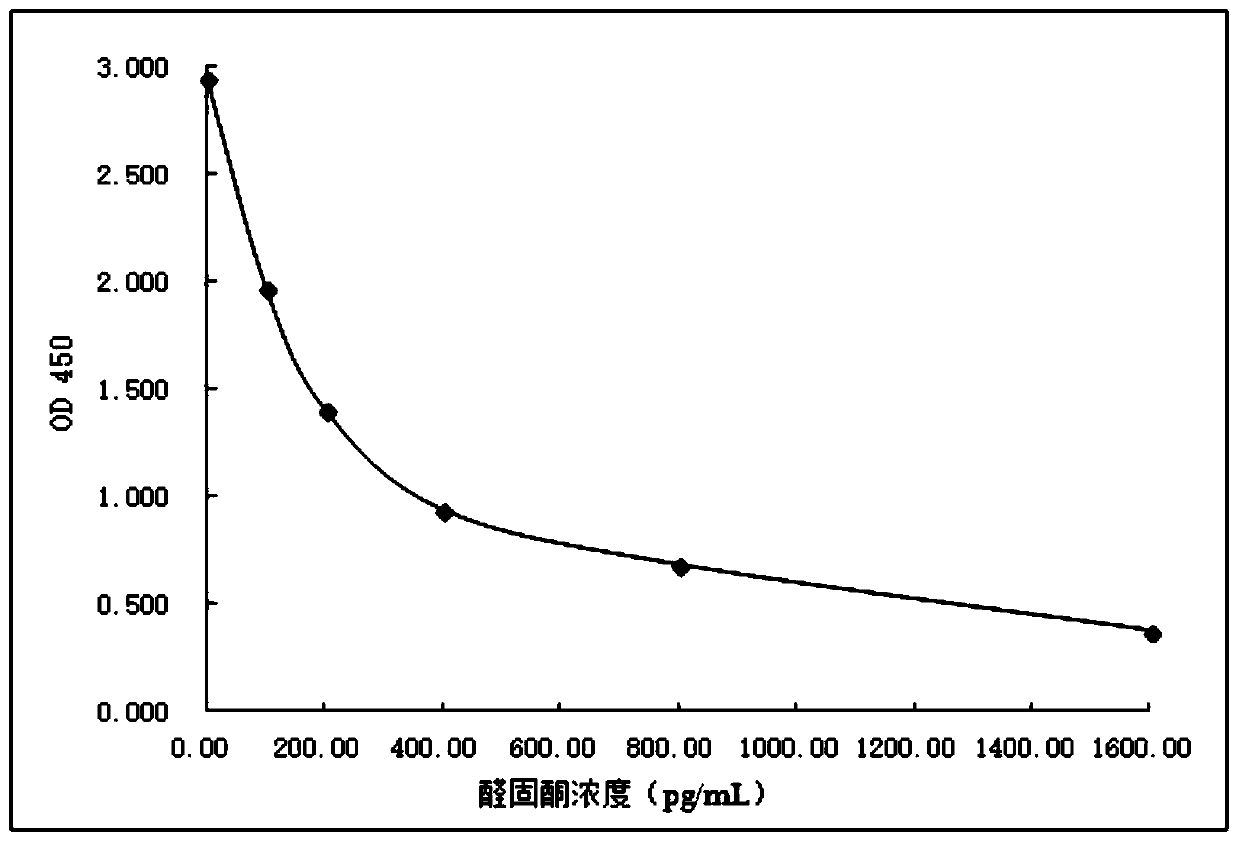
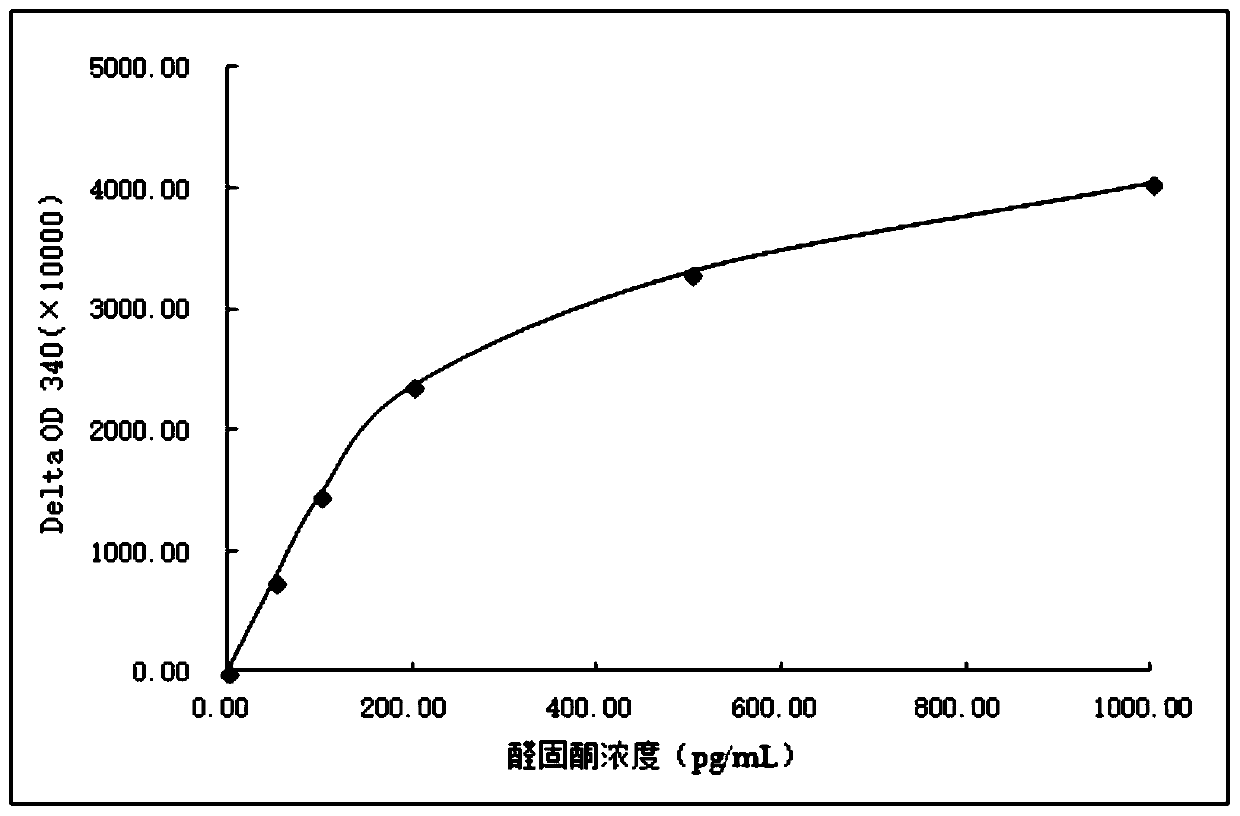
Image
Examples
preparation example Construction
[0058] The synthetic method of aldosterone derivative comprises:
[0059] The carboxyl hydroxyl group of aldosterone is halogenated to obtain a halide. Using the halide as the substrate, the primary amine halogenation reaction with tert-butyl 4-aminobutyrate gives intermediate products containing secondary amine groups. After protecting the secondary amine group in the intermediate product, hydrolysis of the ester group occurs under acidic conditions.
[0060] In the halogenation reaction of the carboxyl hydroxyl group of aldosterone, the halogenation reagents used in the halogenation reaction are lithium chloride (LiCL) and methanesulfonyl halide (MsCL). Aldosterone first reacts with MsCL, and Ms- replaces the hydroxyl hydrogen in the carboxyhydroxyl group of aldosterone. Then, the chlorination reaction with LiCL was carried out.
[0061] When protecting the secondary amine group in the intermediate product, Boc anhydride is used as the protecting agent in the embodiment o...
Embodiment 1
[0128] 1. Synthesis of aldosterone derivatives.
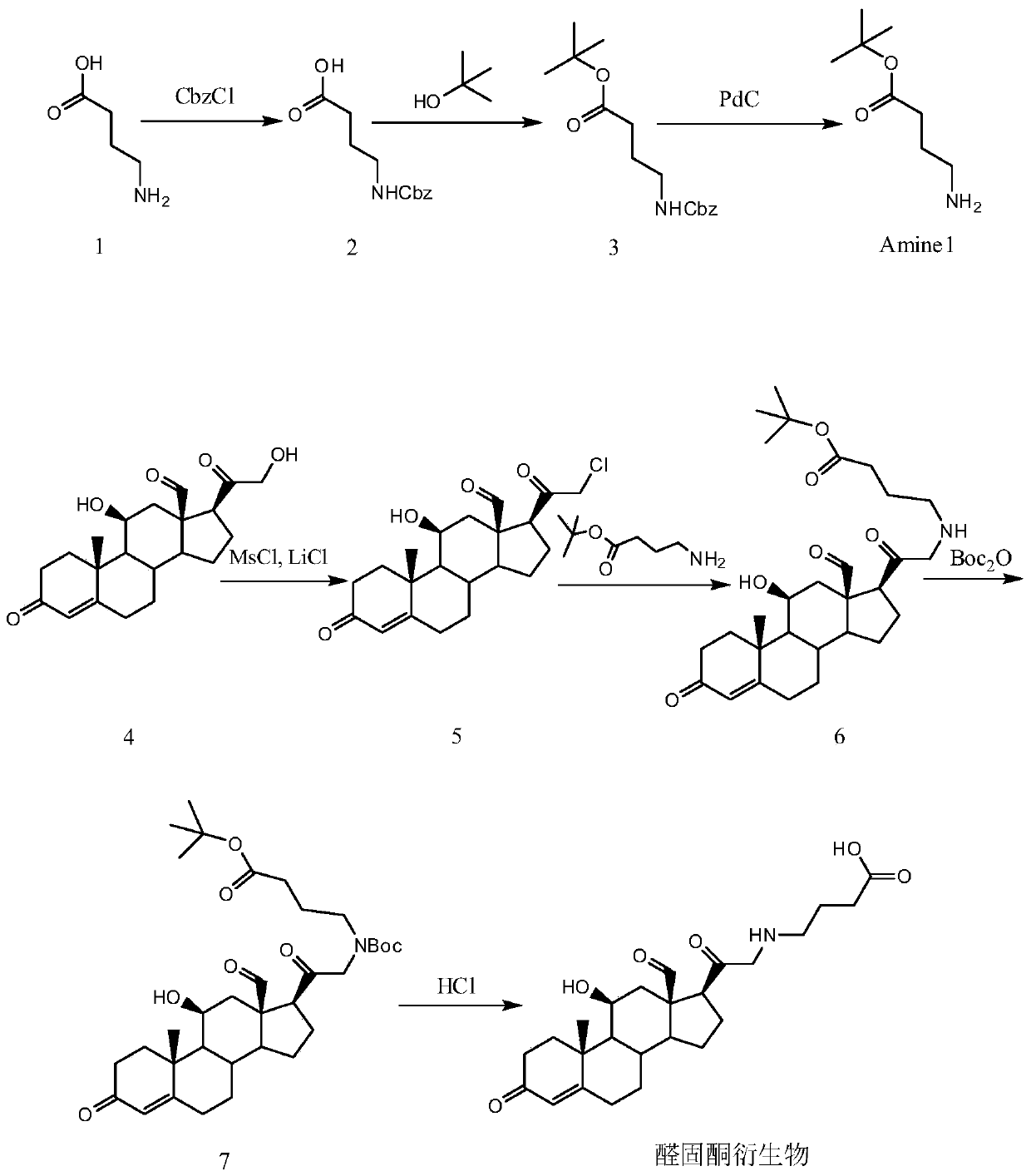
[0129] The synthetic route of aldosterone derivatives is as follows figure 1 shown.
[0130] Wherein, compound 2 is synthesized as follows.
[0131]
[0132] Under the condition of 10°C, 55 grams of catalyst (Cbz-Cl, benzyl chloroformate) was added at one time in the sodium hydroxide solution of 2N normality (2mol / L) dissolved with 30 grams of compound 1 (4-aminobutyric acid) . The mixture was stirred overnight at room temperature. During this period, the reaction process was monitored by TLC until the reaction was complete. The reacted mixture was extracted with ethyl acetate (3x300 mL, 3 times, 300 mL each time). The aqueous layer was adjusted to pH=3 with 1N normal hydrochloric acid and extracted with dichloromethane (3x300 mL). The organic layers were combined, washed with water (200 mL), brine (200 mL), dried in vacuo, and concentrated to obtain compound 2 (33 g, yield 48.8%) as a white solid.
[0133] Wherein, c...
Embodiment 2
[0155] Synthesis of the aldosterone immunogen.
[0156] The aldosterone immunogen is composed of bovine serum albumin (Bovine Serum Albumin, BSA) and -NH-(CH 2 ) 3 The -CO- group is connected. The specific steps of the synthetic method of aldosterone immunogen are as follows:
[0157] 1. Dissolve 200mg of bovine serum albumin in 50ml of 0.2M, pH 8.5 phosphate buffer.
[0158] 2. Add the following chemicals into a small beaker and stir to dissolve: 200mg of the aldosterone derivative synthesized in Example 1, 3.5ml of dimethylformamide, 3.5ml of ethanol, 7.0ml of 10mM potassium phosphate buffer at pH 5.0, 200mg of 1 -Ethyl-3-(-3-dimethylaminopropyl) carbodiimide, 50 mg of N-hydroxysulfosuccinimide, these chemicals were stirred and dissolved at room temperature for 30 min.
[0159] 3. The dissolved solution was added dropwise to the BSA solution, and stirred overnight at 2-8° C. to obtain the antigen; the synthesized antigen was purified by dialysis to obtain the aldosterone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com