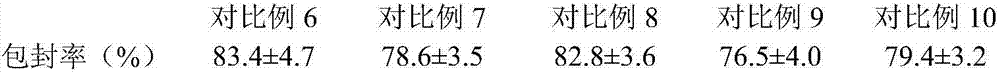Topiroxostat microspherical preparation and preparation method thereof
A technology of topicastat and microspheres, applied in the field of topicastat microsphere preparations and their preparation, can solve the problems of limited clinical application, inconvenient taking, etc., and achieves increased route of administration, stable quality, and improved compliance. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
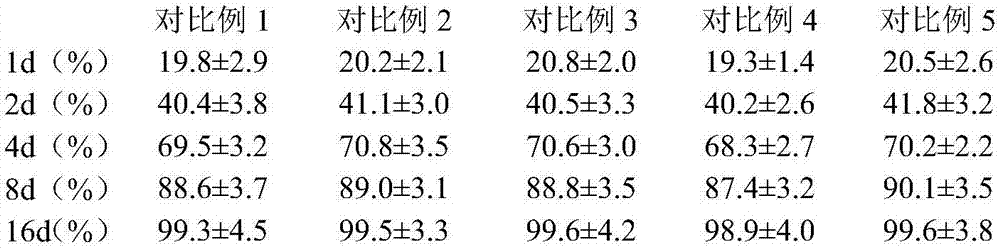
[0023] Weigh 10g of topinostat, dissolve in 20mL of dichloromethane as the dispersed phase; weigh 40g of polylactic acid hydroxylactic acid and 3g of Span 80, dissolve in 100mL of ethanol aqueous solution as the continuous phase, slowly drop the dispersed phase into the organic phase Emulsify at 30°C and 2000r / min for 20min, pour the obtained emulsion into 400ml of acetone to solidify, wash the sediment with acetone until there is no oil drop; prepare 5% mannitol solution, take 100mL and add it to the sediment , stir evenly, and vacuum freeze-dry. The vacuum freeze-drying process is to pre-freeze at -40°C for 2 hours, slowly heat up to -5°C for 10 hours in vacuum, slowly heat up to 10°C for 8 hours in vacuum, and continue to heat up to 30°C for 1 hour in vacuum. The encapsulation rate of topicastat in the microspheres was 88.2±4.1%, and the particle size was 0.73±0.02 μm.
Embodiment 2
[0025] Weigh 20g topinostat, dissolve it in 20mL chloroform as the dispersed phase; weigh 80g chitosan and 2g sodium lauryl sulfate, dissolve it in 200mL ethanol aqueous solution as the continuous phase, slowly drop the dispersed phase into the organic phase, ultrasonically emulsified for 30 minutes to form an oil-in-water emulsion; the organic solvent was removed by rotary evaporation at 40°C, 100 mL of 3% glucose solution was added, and vacuum freeze-drying was obtained. The vacuum freeze-drying process is pre-freezing at -30°C for 4 hours, slowly raising the temperature to 3°C for 12 hours in vacuum, slowly raising the temperature to 15°C for 6 hours, and continuing to heat up to 32°C for 2 hours in vacuum. The encapsulation rate of topicastat in the microspheres was 92.5±3.7%, and the particle size was 0.54±0.04 μm.
Embodiment 3
[0027] Weigh 15g of topinostat and dissolve it in 20mL of dichloromethane; weigh 100g of polylactic acid and dissolve it in 200mL of ethanol aqueous solution, mix the two solutions and spray dry to prepare the topinostat microsphere preparation. The spray drying conditions are: atomization pressure 15MPa, feed liquid temperature 50°C, hot air temperature in the spray drying tower 110°C, feed pump speed 5r / min. The encapsulation rate of topicastat in the microspheres was 80.8±2.9%, and the particle size was 0.96±0.07 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com