Preparation method of high-capacity iron-based lithium ion battery cathode material alpha-LiFeO2
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as poor electrochemical performance, complicated synthesis methods, and impure products, and achieve single phase and good electrochemical performance , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
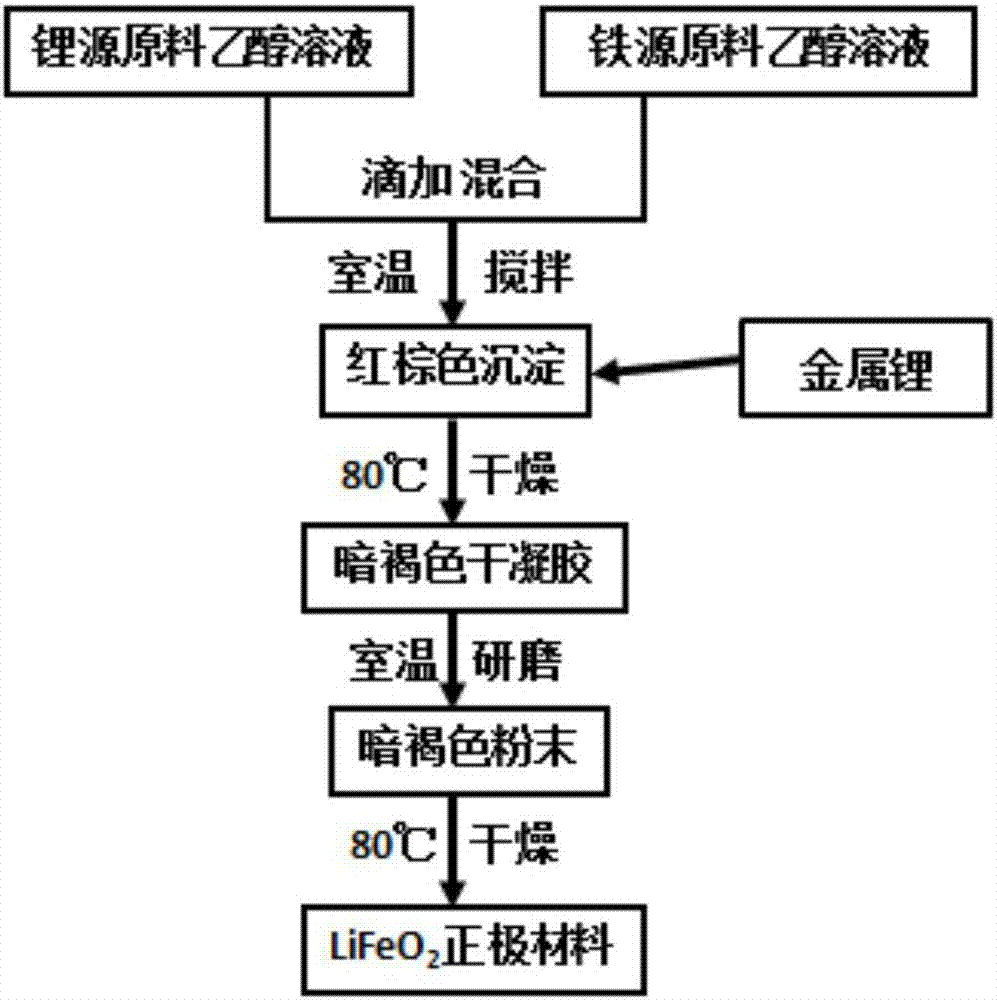
[0031] Dissolve 0.035mol (1.473g) of lithium hydroxide monohydrate and 0.004mol (1.616g) of ferric nitrate nonahydrate in an appropriate amount of absolute ethanol, and add the ferric nitrate solution dropwise to lithium hydroxide at room temperature and under magnetic stirring. Add 0.004mol (about 0.028g) lithium metal to the solution, and stir magnetically at room temperature for 5 hours to obtain a dark brown-brown precipitate; after centrifugation, wash repeatedly with absolute ethanol and deionized water for 5 times, and finally Wash with absolute ethanol to remove excess Li + and other impurity ions, and then put the final sample washed with absolute ethanol in a blast drying oven at 80°C for 4 hours, then manually grind the dried sample for 20 minutes, and then put the refined powder into an 80°C drying oven again Continue to dry for 20h in the middle to obtain lithium-rich layered α-LiFeO 2 Lithium-ion battery cathode material.
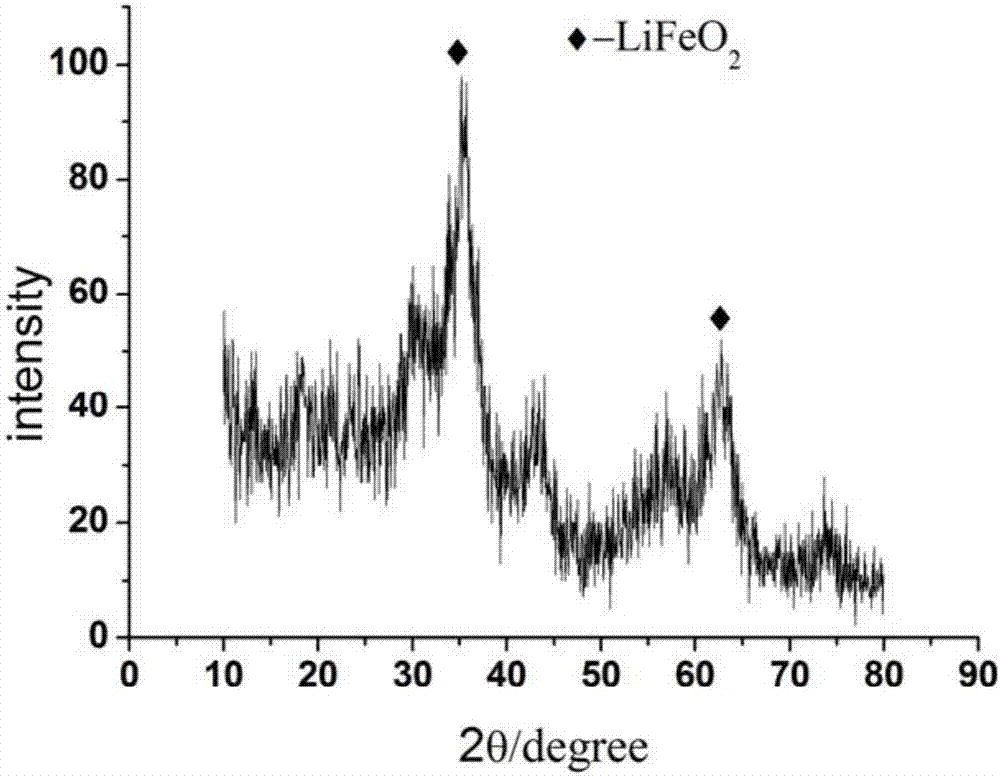
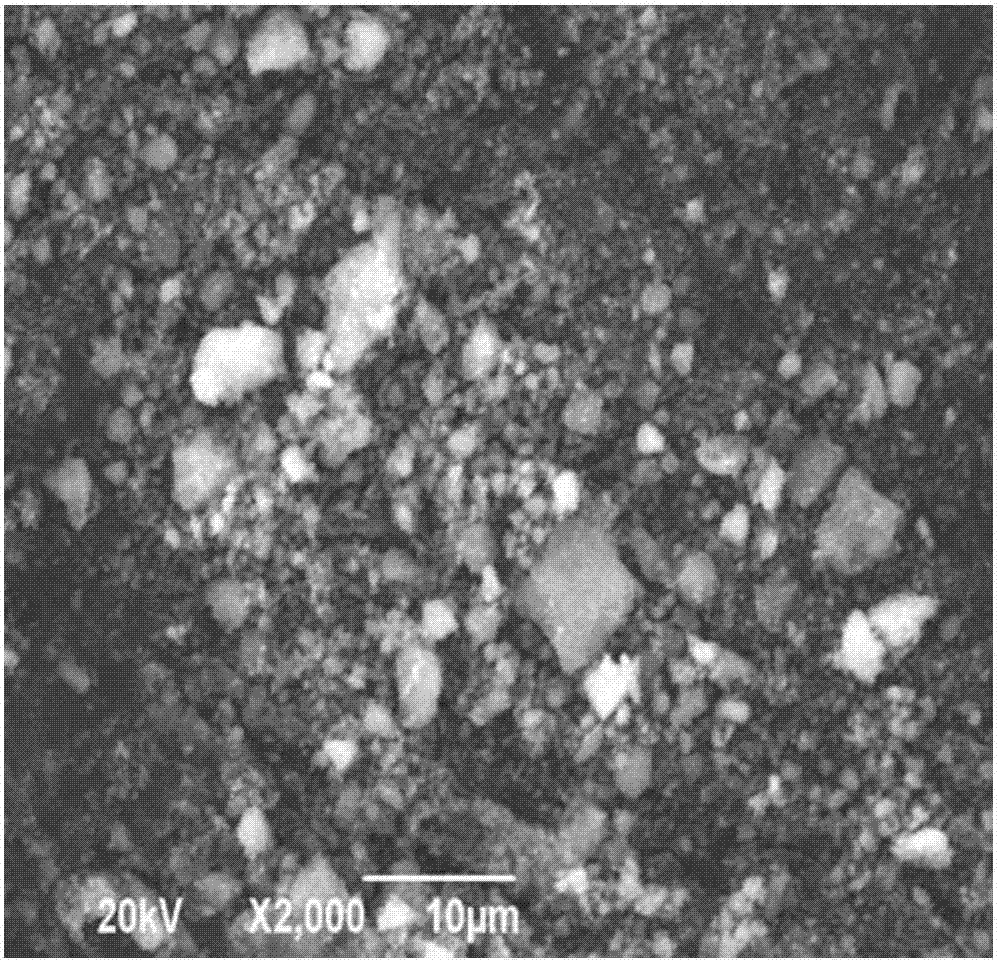
[0032] For the above α-LiFeO 2 Lithi...
Embodiment 2
[0034] Dissolve 0.109mol (7.211g) of anhydrous lithium acetate and 0.012mol (5.05g) of ferric nitrate nonahydrate in an appropriate amount of absolute ethanol, and add the ferric nitrate solution dropwise to anhydrous lithium acetate at room temperature and under magnetic stirring. Add 0.012mol (about 0.084g) lithium metal to the solution, and stir magnetically at room temperature for 5 hours to obtain a dark brown-brown precipitate. After centrifugation, wash with absolute ethanol and deionized water alternately and repeatedly for 5-8 times. to remove excess Li + and other impurity ions, and then put the final sample washed with absolute ethanol in a blast drying oven at 80°C for 4 hours, then manually grind the dried sample for 20 minutes, and then put the refined powder into an 80°C drying oven again Continue to dry for 20h in the middle to obtain lithium-rich layered α-LiFeO 2 Lithium-ion battery cathode material. Use it as a positive electrode active material, mix it wi...
Embodiment 3
[0036] Dissolve 0.048mol (2.016g) of lithium hydroxide monohydrate and 0.004mol (1.616g) of ferric nitrate nonahydrate in an appropriate amount of absolute ethanol, and add the ferric nitrate solution dropwise to hydrogen monohydrate at room temperature and under magnetic stirring. Add 0.004mol (about 0.028g) lithium metal to the lithium oxide solution, and continue magnetic stirring at room temperature for 5 hours to obtain a dark brown precipitate. After centrifugation, wash repeatedly with absolute ethanol and deionized water alternately. to remove excess Li + and other impurity ions, and then the final sample washed with absolute ethanol was placed in a blast drying oven at 80°C for 6 hours, and then the dried sample was manually ground for 20 minutes, and the obtained fine powder was placed in an 80°C drying oven again Continue to dry for 16h in the middle to obtain lithium-rich layered α-LiFeO 2 Lithium-ion battery cathode material. Use it as a positive electrode activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com