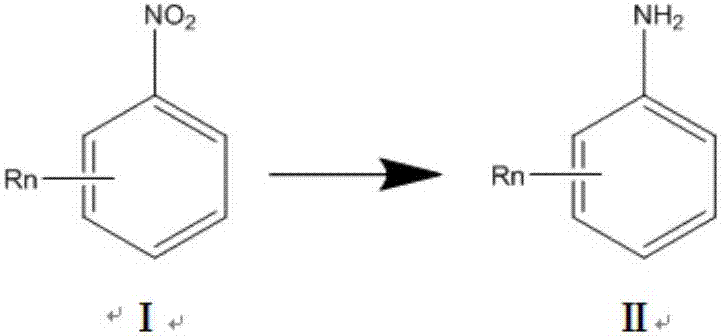
Supported noble-metal hydrogenation catalyst, and preparation method and application of catalyst
A hydrogenation catalyst and precious metal technology, applied in the fields of hydrogenation catalyst and its preparation and application, can solve problems such as retention, and achieve the effects of fast filtration, simplified process flow and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
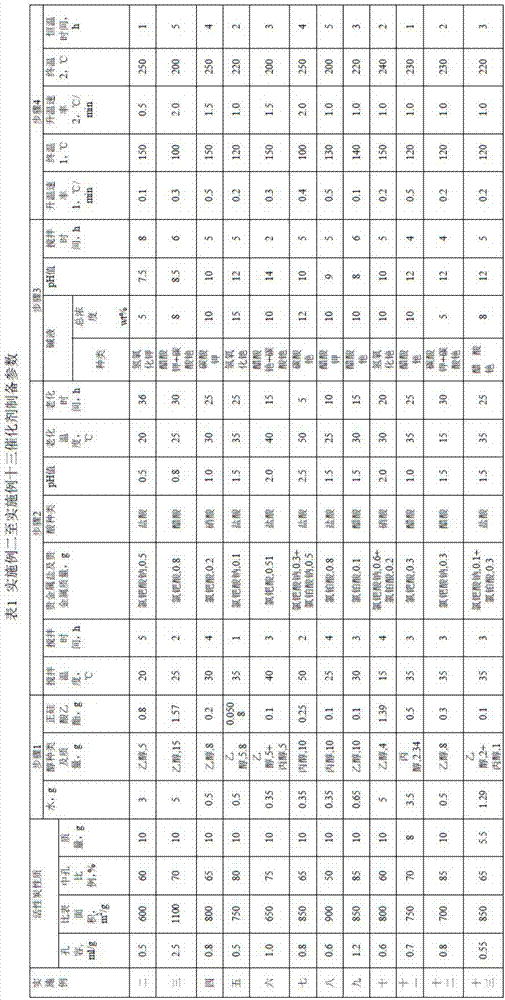
[0054] Weigh 10g of activated carbon, the specific surface area of activated carbon is 1000m 2 / g, the proportion of mesopores is 50%, and the pore volume is 0.8mL / g. Weigh 1g of water, 8g of ethanol, and 0.1g of ethyl orthosilicate in a beaker, and stir well until completely dissolved. Activated carbon was poured into the mixture and stirred at 25°C for 2 hours. Then chloroplatinic acid containing 0.2 g of platinum was added, followed by adjusting the pH of the activated carbon slurry to 1.0 with hydrochloric acid, and aging at 25° C. for 20 hours. Then adjust the pH of the activated carbon slurry to 9.0 with 5 wt% potassium hydroxide solution, continue aging at constant temperature for 5 hours, filter, wash with ethanol until the pH of the filtrate reaches neutral, and obtain a catalyst precursor. Finally, in a hydrogen atmosphere, the room temperature was raised from room temperature at 0.1°C / min (heating rate 1) to 150°C (final temperature 1), and then at 0.5°C / min (he...
Embodiment 14
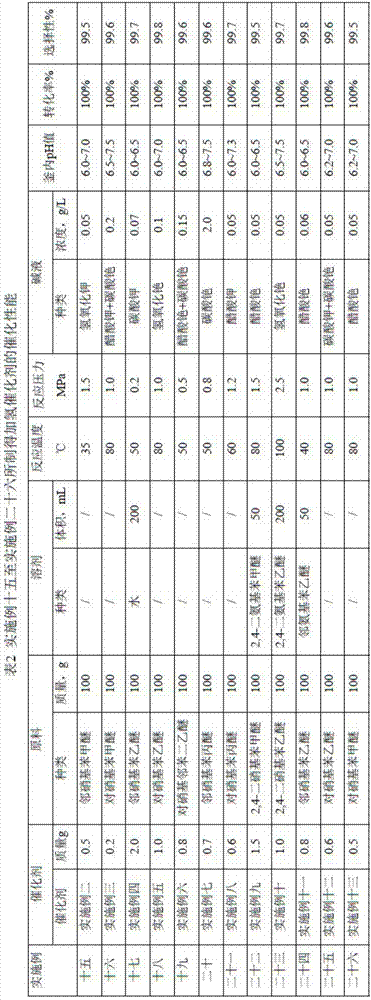
[0060]Add 100g of p-nitrophenethyl ether and 0.8g of the catalyst of Example 1 into a 500mL autoclave, close the autoclave, replace the air in the reactor with nitrogen, then replace the nitrogen with hydrogen, start stirring, and the stirring speed is 1400r / min. The reaction was carried out by maintaining the reaction temperature at 50° C. and the hydrogen pressure at 1.0 MPa. During the reaction, the pH value of the hydrogenation solution is monitored online in real time, and the cesium hydroxide lye is adjusted in real time to keep the pH value of the hydrogenation solution in the kettle at 6.0-7.5, and control the concentration of cesium ions in the hydrogenation solution to 0.5g / L. When the content of p-nitrophenethyl ether was detected to be 0 by chromatography, the reaction was stopped and the catalyst was filtered. The filtrate is the product after phase separation, water separation and dehydration by vacuum distillation. Quantitative analysis (molar percentage) by c...
Embodiment 27
[0068] Embodiment 27 is the catalyst application experiment of Embodiment 14, and the results are shown in Table 3.
[0069]
[0070]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com