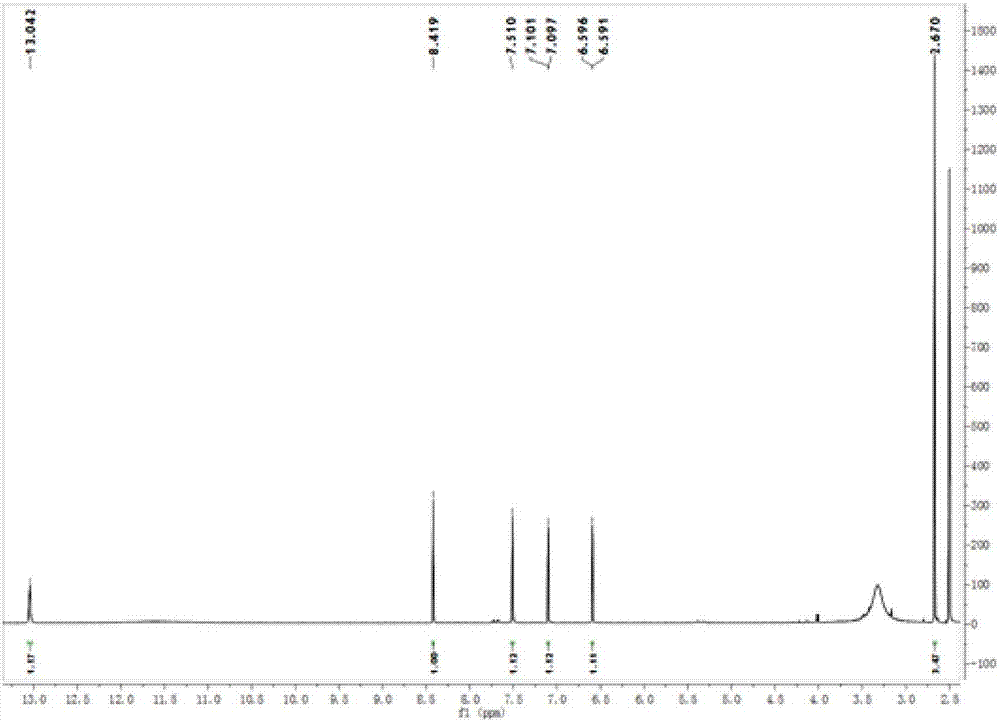
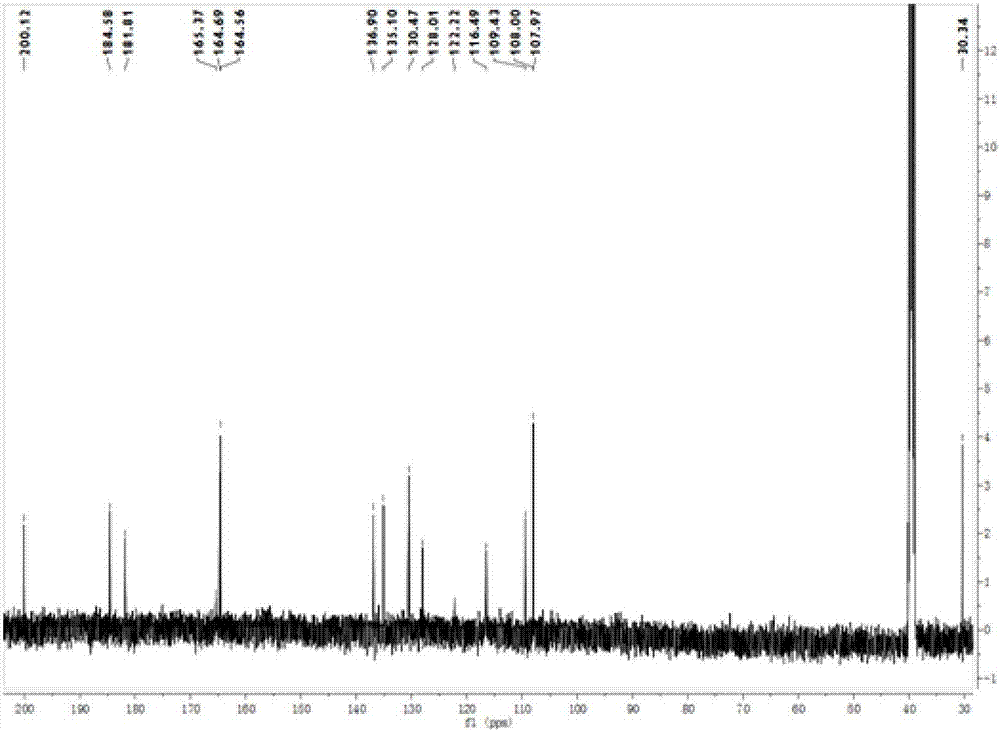
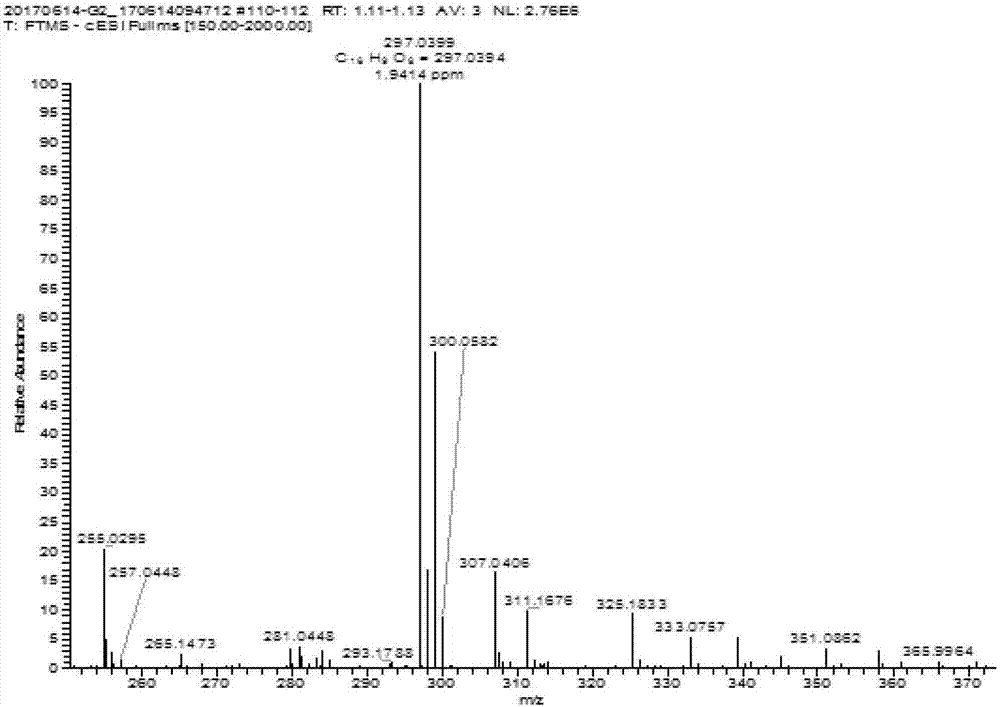
Anthraquinone compounds, preparation method and application
A compound and anthraquinone technology, applied in the field of anthraquinone compound preparation, can solve the problems of no marine fungal secondary metabolites for preventing and controlling plant bacterial diseases, less application, etc., achieve excellent bactericidal performance, not limited by resources, preparation Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Another embodiment of the present invention provides a method for preparing anthraquinone compounds, comprising the following steps:
[0042] The marine fungus Fusarium equiseti GLY27 was cultured in the strain culture medium, and then transferred to the fermentation medium for fermentation;
[0043] Soak the obtained fermented product in ethyl acetate, then soak it in a solution with a volume ratio of methanol:dichloromethane of 1:1, concentrate under reduced pressure, filter, extract the remaining water phase with ethyl acetate, concentrate the extract under reduced pressure, and dry After the crude extract;
[0044] The crude extract is subjected to chromatography, elution, and concentration to obtain the compound described in formula (I).
[0045] In this example, marine fungi can produce metabolites after strain activation and fermentation expansion, which is beneficial to the preparation of extracts. After fermentation, the fermentation products are soaked in eth...
Embodiment 1
[0056] The cultivation and fermentation of embodiment 1 marine fungus Fusarium equiseti GLY27
[0057] The strain culture of marine fungus Fusarium equiseti GLY27 adopts potato dextrose agar medium, which contains potato 6g / L, glucose 20g / L, agar 20g / L, and the rest is water. 5-7 days;
[0058] (2) Fermentation culture of marine fungus Fusarium equiseti GLY27 Use rice solid medium, add rice 40g, water 30mL to each 500mL Erlenmeyer flask, ferment 3 bottles, and culture the fungal strain at 28°C for 30-40 days.
Embodiment 2
[0059] The preparation of embodiment 2 anthraquinone compounds
[0060] Soak the fermented product obtained twice in ethyl acetate, then soak in a solution with a volume ratio of methanol:dichloromethane of 1:1, concentrate under reduced pressure, filter, extract the remaining aqueous phase with ethyl acetate, and concentrate the extract under reduced pressure , to obtain the crude extract after drying;
[0061] The crude extract was subjected to vacuum silica gel column chromatography, the stationary phase was 100-200 mesh normal phase silica gel, the mobile phase was eluted with 10% ethyl acetate-petroleum ether mixed solvent, and then eluted with 100% ethyl acetate. After the 100% ethyl acetate eluate was concentrated, ODS (48-63 μ m) reversed-phase silica gel column chromatography was carried out, and the mobile phase was 30%, 40%, 50%, 60%, 70%, 80% aqueous methanol, 50 After concentration of the % methanol eluate, Sephadex LH-20 gel column chromatography was carried out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com