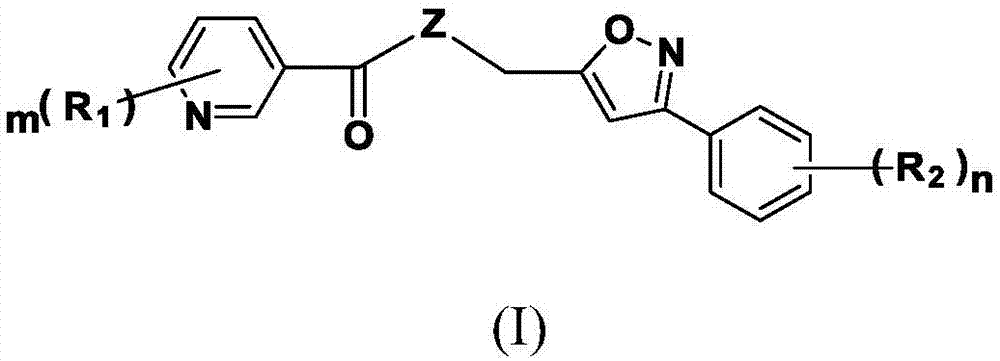
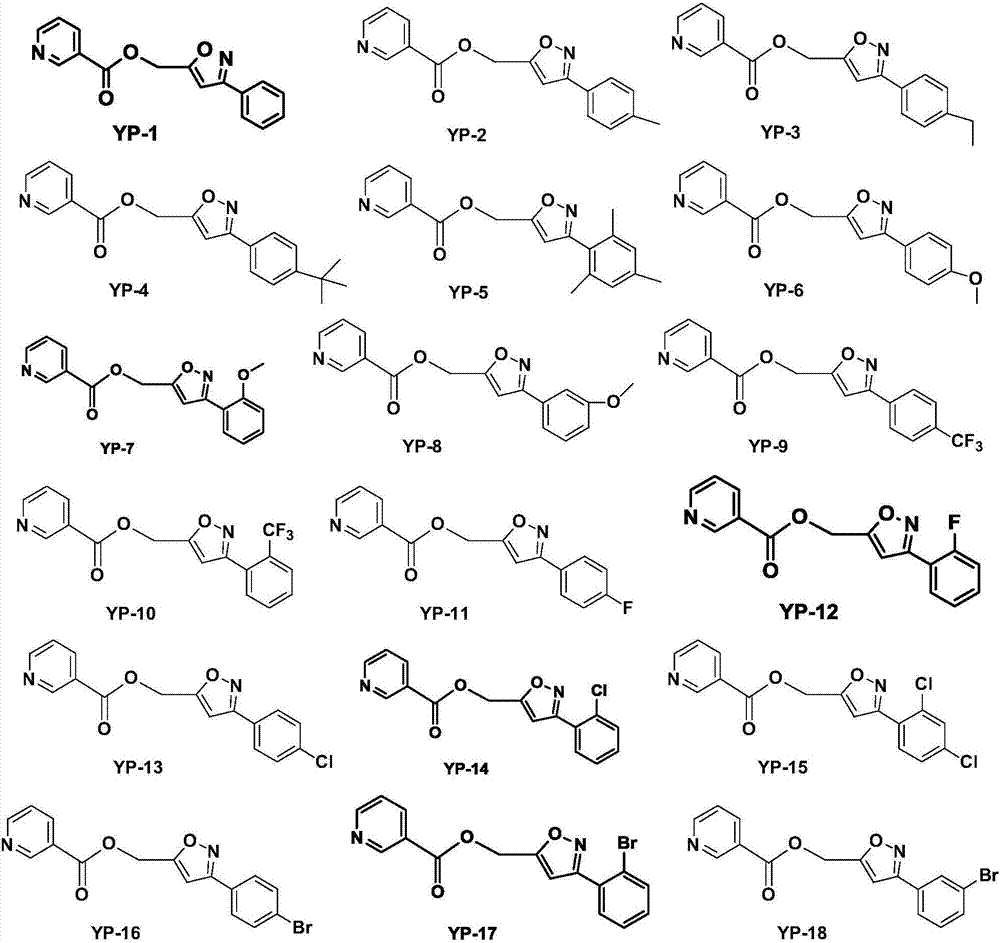
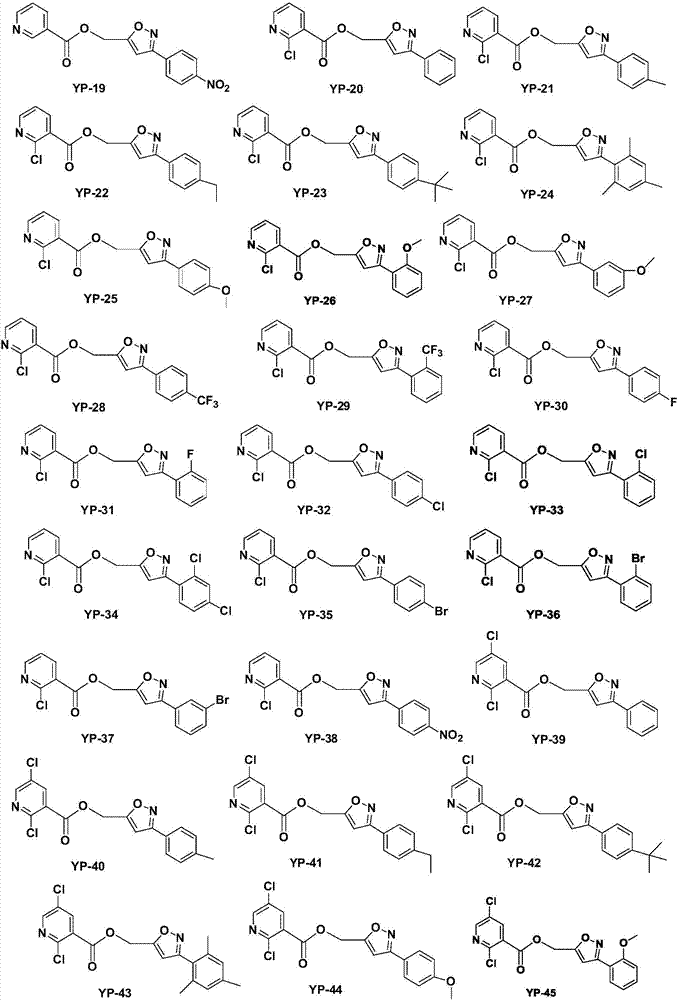
Nicotinic acid derivatives and preparation method and applications thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve problems such as infertility, affecting physiological functions, and damaging normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0070] Synthesis of Preparation Example 1 intermediate (II-1) and intermediate (II-2):
[0071] Using substituted benzaldehyde as raw material, it is prepared by synthesis of oxime, 1,3-dipolar cycloaddition reaction, methylsulfonyl esterification reaction, azidation, and reduction reaction. See the following process for details:
[0072]
[0073] For the specific synthesis process of intermediate (II-1) and intermediate (II-2), please refer to Chinese patent applications CN103360382A, CN103664991A and CN103601762A, and these three applications are fully incorporated into the present invention.
[0074] According to the present invention, for compounds where Z is other substituents, such as NR 3 Or S, can use the corresponding substituted aminopropyne compound or propyne thiol as raw material, according to the preparation process of intermediate (II-2) to prepare.
Embodiment 1
[0075] Synthesis of niacin derivatives shown in embodiment 1 formula (I)
[0076] Among them, the reaction of nicotinic acid and 3-phenyl-5-hydroxymethyl-isoxazole is exemplified:
[0077] Synthesis of [(3-phenyl-isoxazol-5-yl)-methyl]-pyridine-3-carboxylate (YP-1)
[0078]
[0079] Add 0.123g (1mmol) of nicotinic acid and 0.206g (1mmol) of DCC into a 50mL round bottom flask, add 10mL of dry THF, stir in an ice bath for 30min, and dissolve 0.175g (1mmol) of 3-phenyl-5-hydroxy A 10 mL THF solution of methyl-isoxazole and 0.122 g (1 mmol) DMAP was slowly added dropwise to the reaction system, stirred in an ice bath for 30 min, and then naturally rose to room temperature for reaction. After the completion of the TLC detection reaction, the reaction solution was concentrated in vacuo, and the residue was directly separated from the column. (石油醚) :V (乙酸乙酯) =5:1~2:1), the target compound [(3-phenyl-isoxazol-5-yl)-methyl]-pyridine-3-carboxylate (YP-1) was obtained. Other compo...
Embodiment 2
[0080] Synthesis of nicotinic acid amide derivatives shown in embodiment 2 formula (I)
[0081] Wherein the reaction of nicotinic acid and 3-phenyl-5-aminomethyl-isoxazole is exemplified:
[0082] Synthesis of N-[(3-phenyl-isoxazol-5-yl)-methyl]-pyridine-3-carboxamide (YP-115)
[0083]
[0084] Add 0.123g (1mmol) nicotinic acid, 0.206g (1mmol) DCC and 0.135g (1mmol) HOBT into a 50mL round bottom flask, add 10mL dry THF, stir in an ice bath for 30min, and dissolve 0.174g (1mmol) 3 -Phenyl-5-aminomethyl-isoxazole and 0.122 g (1 mmol) DMAP in 10 mL THF were slowly added dropwise to the reaction system, stirred in an ice bath for 30 min, and then naturally rose to room temperature for reaction. After the completion of the TLC detection reaction, the reaction solution was concentrated in vacuo, and the residue was directly separated from the column. (石油醚) :V (乙酸乙酯) =5:1~2:1), the target compound N-[(3-phenyl-isoxazol-5-yl)-methyl]-pyridine-3-carboxamide (YP-115) was obtained....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com