Phenylboronic acid functionalized zwitterion blocked copolymer and glucose-sensitive bionic nano-carrier
A block copolymer and glucose-sensitive technology, which can be used in drug combinations, medical preparations of non-active ingredients, metabolic diseases, etc., and can solve problems such as limitations in application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
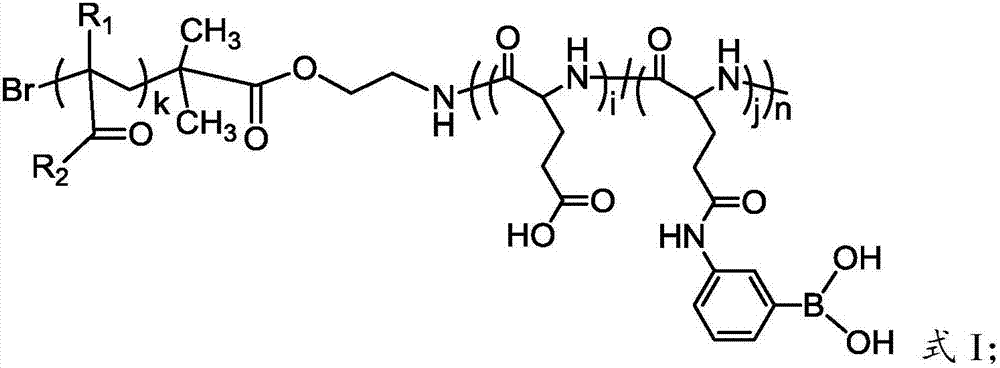
[0107] In a 10mL single-necked flask equipped with a magnet, add the initiator of the formula V structure, carboxybetaine methyl acrylate (CBMA) monomer (9.17g, 40mmol) and 90mL H 2 O / CH 3 OH (1:l, v / v) mixed solvent. After stirring and fully dissolving, under the protection of nitrogen, after three cycles of freezing, pumping, and melting, CuBr (7.2mg, 0.05mmol) and BPY (16mg, 0.1mmol) that had been deoxygenated were added, and reacted at 35°C for 48h. After the reaction, the solution was exposed to air to terminate the reaction. The resulting reaction solution was passed through a silica gel column using methanol as an eluent to remove copper salts and BPY. The filtrate was concentrated by rotation, settled with ether, filtered, and dried in vacuum to obtain a Boc-protected zwitterionic polymer.
[0108] The Boc-protected zwitterionic polymer was dissolved in 50 mL of DMF, and 16 mL of trifluoroacetic acid solution (trifluoroacetic acid / dichloromethane: 25%, v / v) was adde...
Embodiment 2
[0113] In a 10mL single-necked flask equipped with a magnet, add the initiator of formula V structure (310.18mg, 1mmol), thiobetaine methyl acrylate (SBMA) monomer (22.35g, 80mmol) and 200mL H 2 O / CH 3 OH (1:l, v / v) mixed solvent. After stirring and fully dissolving, under the protection of nitrogen, after three cycles of freezing, pumping, and melting, CuBr (7.2mg, 0.05mmol) and BPY (16mg, 0.1mmol) that had been deoxygenated were added, and reacted at 35°C for 48h. After the reaction, the reaction solution was exposed to air to terminate the reaction. The reaction solution was passed through a silica gel column using methanol as an eluent to remove copper salts and BPY. The filtrate was concentrated by rotation, settled with ether, filtered, and dried in vacuum to obtain a Boc-protected zwitterionic polymer.
[0114] The Boc-protected zwitterionic polymer was dissolved in 100 mL of DMF, and 16 mL of trifluoroacetic acid solution (trifluoroacetic acid / dichloromethane: 25%, ...
Embodiment 3
[0119] In a 10mL single-necked flask equipped with a magnet, add the initiator of the formula V structure (310.18mg, 1mmol), phosphorylcholine methacrylate (MPC) monomer (33g, 110mmol) and 300mL H 2 O / CH 3 OH (1:l, v / v) mixed solvent. After stirring and fully dissolving, under the protection of nitrogen, after three cycles of freezing, pumping, and melting, CuBr (7.2mg, 0.05mmol) and BPY (16mg, 0.1mmol) that had been deoxygenated were added, and reacted at 35°C for 48h. After the reaction, the solution was exposed to air to terminate the reaction. The reaction solution was passed through a silica gel column using methanol as an eluent to remove copper salts and BPY. The filtrate was concentrated by rotation, settled with ether, filtered, and dried in vacuum to obtain a Boc-protected zwitterionic polymer.
[0120] The Boc-protected zwitterionic polymer was dissolved in 150 mL of DMF, and 16 mL of trifluoroacetic acid solution (trifluoroacetic acid / dichloromethane: 25%, v / v) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com